MINI-REVIEW
Brianda Barrios-Lopez1,3, Anu Airaksinen1, Kim Bergström2,3,4,*
1Laboratory of Radiochemistry, Faculty of Chemistry, University of Helsinki, Finland
2Center for Drug Research, Faculty of Pharmacy, University of Helsinki, Finland
3Division of Pharmacology and Toxicology, Faculty of Pharmacy, University of Helsinki, Finland
4HUS Medical Imaging Center, Helsinki University Central Hospital, Finland
Table of Contents
Abstract
Alzheimer’s disease (AD) is a brain disorder with aggravating symptoms of memory loss and dementia mainly in the elderly population. Imaging tools of β-amyloid (Aβ) plaques are necessary for clinical and neuropsychological characteristics in AD. This is in order to gain global understanding of the disease by the design and selection of effective drugs. Positron emission tomography (PET) is a notable tool for drug advancement because of its high sensitivity and capacity to produce quantitative and kinetic data. PET amyloid imaging is an appropriate technique for studying the amyloid binding. Currently, Pittsburgh P (11C-PIB), a benzothiazole compound, is an outstanding tracer used for attachment to the Aβ plaques. Other PET imaging probes already in use in clinical trials include thioflavin T, 11C-SB-13, 18F-GE-067,18F-AZD4694, 18F-BAY94-9172 and 18F-AV-45. PET imaging has received prominence because of these important advances in the in vivo β-amyloid plaques detection. However, there is still a need for better tracers. A pathway not yet developed is PET tracers with 68Ga chemistry. 68Ga decays by 89% over positron emission of 1.9 MeV and 11% orbital electron capture. 68Ga possesses a half-life (Τ1/2) of 67.7 min. An important advantage is the long half-life of the generator 68Ge/68Ga generator system that is 270 days. This review focuses on the use of gallium-68 radiotracers used for human amyloid imaging.
Keywords: Alzheimer’s disease; PET amyloid imaging; diagnostic imaging
1. Introduction
1.1 Alzheimer’s disease and PET amyloid imaging
Alzheimer’s disease (AD) is a brain disorder with aggravating symptoms of memory loss and dementia mainly in the elderly population. The two remarkable signs of AD are the presence of β-amyloid (Aβ) plaques and neurofibrillary tangles (NFTs); these are formed by abnormal hyperphosphorylated insoluble form of the τ-protein. The Aβ plaques appear prior to the behavioural symptoms, and medical and psychiatric evaluations are made [1].
The 2010 World Alzheimer report stipulated that around 36 million people in the world are presenting with AD. This equates to a spend of US$600 billion for treatment. Significantly, the report indicates that the number of cases is going double in 20 years [2].
At present, there is no exact information about the relationship influence between Aβ plaques and NFTs. There are two leading theories:
(a) the amyloid cascade hypothesis which stipulates that the hyperphosphorylation of τ-protein is triggered by the aggregation of Aβ oligomers;
(b) τ and tangle hypothesis which says that a defect in the τ-protein produces the accumulation of Aβ plaques [3].
An important objective in the treatment of AD is an early diagnosis which permits intervention when patients have nominal damage. Equally, examination of Aβ formation and deposition over time to check the progression of the disease as well as to check the effectiveness of the anti-Aβ therapy in use is essential. Therapies that are being developed for AD treatments are focusing on preventing the accumulation of Aβ plaques and tangles or dissolve or break them once they are present [4]. There is an enormous demand for compelling therapies for AD. Right now, there are two ways to treat the symptomatic effects of this disease which are cholinesterase inhibitors and N-methyl-aspartate (NMDA) receptor antagonists. However, these pathways do not avoid the advancement of AD [5].
Imaging tools of Aβ plaques and clinical and neuropsychological characteristics in AD are necessary to obtain a global understanding of the disease and to design and select effective drugs [6]. Molecular imaging has the promise to detect disease evolution and/or therapeutic performance over other techniques. Nearly any molecular imaging technique such as positron emission tomography (PET), single photon emission computed tomography (SPECT), ultrasonic imaging (US) and magnetic resonance imaging (MRI) can be appropriated for quantitative assessment of radiotracers. In addition to drug pharmacokinetics and metabolism [7]. PET is an important tool for drug advancement because of its high sensitivity and capacity to obtain quantitative and kinetic data [8].
A crucial point for the achievement of neuroscience imaging is to provide an efficient and dependable delivery of radiotracers to the brain. It is important to note that in AD and senile dementia, the drug delivery route should be based on transport mechanisms across a “relative” (or low damage) normal blood-brain barrier (BBB) with a low-risk resistance. Other pathways to deliver drugs to the central nervous system (CNS) include (A) Direct delivery: this delivery uses biodegradable systems such as nanoparticles and liposomes. In this method, the drug is dissolved, entrapped, adsorbed, attached or encapsulated into the nanoparticle matrix or liposomes.
Nanoparticles and liposomes are very promising vehicles because of their size, hydrophobic and hydrophilic properties and biocompatibility. This approach is used because nanoparticles and liposomes are small in size and can penetrate through to the brain via small capillaries: this allows efficient drug accumulation. In addition, the drug could be released at the target site over a period of days or even weeks; (B) Enhanced delivery through BBB: this delivery works with the lipid solubility and receptor-mediated process: as cerebral microvessels express receptors for low-density lipoproteins, insulin, insulin-like growth factor-I and II, transferrin and leptin.
This approach may be achieved by attachment of the drug to peptide or protein “vectors”, which are transported into the brain from the blood by receptor-mediated transcytosis via the BBB. (C) Targeting technologies: these methods utilise local concentrations using magnetic nanoparticles and immunological targeting. This method relies on the use of magnetic particles bound to a specific antibody. The magnetic immunoassay tool is specialized in measuring the magnetic field generated by the magnetically labelled target(s) which are detected with a sensitive magnetometer. However, this approach has met with little success thus far. Since an intense magnetic field required for selective drug delivery this can only be achieved relatively close to the brain surface and not deep within the brain parenchyma. However, when used in small animals, magnetic targeting can boost regional drug delivery by 50-500% [9–11].
1.2 Diagnostic agents for beta-amyloid imaging using PET tracers
The introduction of 18F-FDG has enormously advanced human PET. Some of the achievements of PET involve the use and the application of 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) in 1976 by Abass Alavi and his group at the University of Pennsylvania. In 1977, Then, Sokoloff et al. measured cerebral glucose metabolism adopting [14C]-2-deoxyglucose. Finally, the fundamental PET/CT scanner prototype was developed by Townsend et al. in Pittsburgh in 1998 [12]. Some PET isotopes commonly used include 18F, 11C, 15O, 82Rb, and in a lesser extent used 68Ga, 64Cu and 89Zr.
18F-FDG possesses a half-life of 110 min and is an established precursor to the synthesis of other labelling probes. The production and synthesis conditions reassure an efficient high radiochemical yield for diagnostic scopes [13]. Many experiments have tested regional cerebral glucose metabolism with 18F-FDG: common results indicate a lower uptake of temporoparietal with lesser uptake in the basal ganglia, thalamus, cerebellum, and primary sensorimotor cortex. These events are indications of AD disease [6].
PET amyloid imaging is an appropriate technique for studying the amyloid binding. Amyloid imaging will afford presymptomatic detection. Amyloid imaging in patients with hereditary traits of AD – including chromosomal aberrations (APP and PS1 mutations) as well as carriers of apolipoprotein APOE ε4 – consequently a high priority [14]. Human amyloid imaging has been accepted by the Food Drug Administration (FDA) for ordinary clinical use. Pittsburgh P (11C-PIB), a benzothiazole compound, is a most outstanding tracer used for attachment to the β-amyloid plaques [15].
Preclinical studies have demonstrated that 11C-PIB targeted to the AD brain with a Kd of 1 nM can pass through the BBB rapidly (7% ID/g 2 minutes after intravenous injection in mice). Furthermore, it is eliminated swiftly from normal mouse brain (clearance t1/2 almost 8 minutes) [16]. However, there are two disadvantages for its commercial use: short half-life of 20 minutes and problematic manufacturing practice [17].
In principle, Aβ imaging probes should: 1) adequately image brain Aβ accumulation; 2) possess an excellent reproducibility in many patients and clinical settings; and 3) have good, cost-effective marketing. Only a few Aβ tracers are achieving these objectives [5]. Imaging tracers are presently used to achieve an appropriate resolution of organs and superior localization of the target due to the accumulation of the imaging agent [18]. A few parameters have to be taken into account while designing imaging agents such as pharmacokinetics, solubility, lipophilicity, efficacy, and toxicity [19].
Currently PET imaging probes already in use in clinical trials are: 11C-SB-13, thioflavin T, 18F-GE-067, 18F-AZD4694, 18F-BAY94-9172, 18F-AV-45 (Table 1) [20]. PET imaging is becoming strengthened with these important advances in the in vivo β-amyloid plaques detection [1].
11C 3H 18F 123/125I
[11C]AZD2184 [3H]AZD2184 [18F]BF-168 [123F]IMPY
[11C]Benzofuran
[18F]BF-227 [125I]TZDM
[11C]BF-145 [18F]FDDNP
[11C]BF-227 [18F]FEM-IMPY
[11C]MES-IMPY [18F]Florbetaben
[11C]PIB [18F]Florbetapir
[11C]SB-13 [18F]Flutemetamol
[18F]FPM-IMPY
Table 1. Examples of tracers used in amyloid imaging.
2. Gallium-68 and chelates
A critical point for the progress in PET tracers includes the development of 68Ga chemistry. Considering the retail possibility of 68Ge/68Ga generators: cyclotron-independent on-site production of 68Ga has become achievable [20]. 68Ga is a positron emitter with a half-life (Τ1/2) of 67.7 min; however, it is fast decaying and might be a possible short-coming for commercial radiopharmaceuticals [21]. 68Ga decays by 89% over positron emission of 1.9 MeV and 11% orbital electron capture. The parent 68Ge is accelerator produced on Ga2O3 targets by a (p, 2n) reaction. It decays with a half-life of 270.8 days by electron capture (Figure 1).
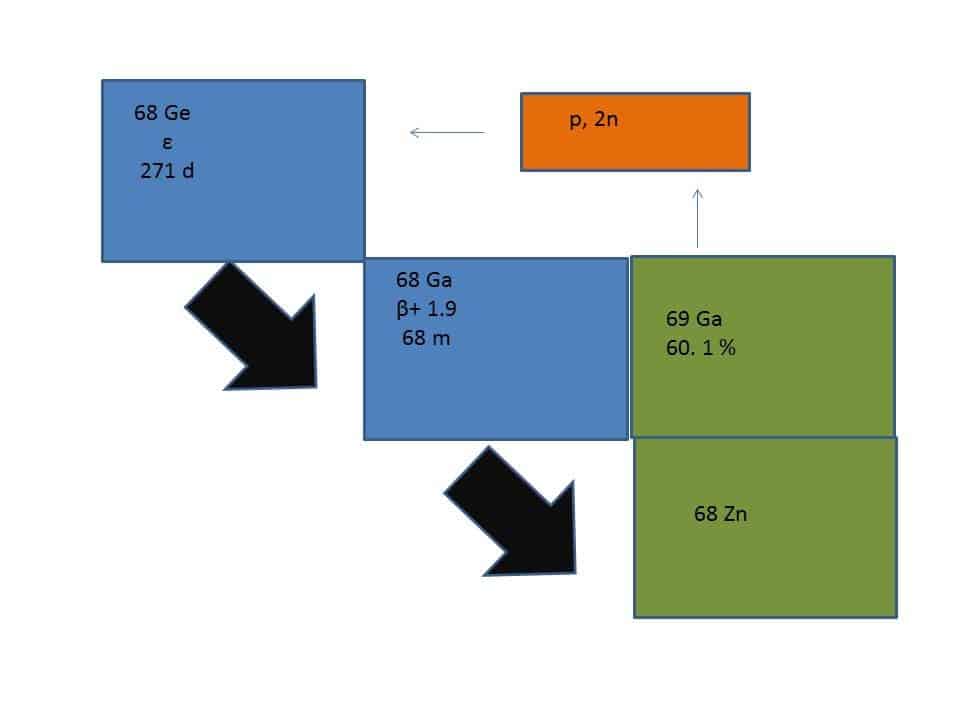
Figure 1. Ge-68/Ga-68 generator.
68Ge is firmly absorbed onto various solid supports such as metal oxides (Al2O3, TiO2, or SnO2) and organic pyrogallol-formaldehyde resins. Ga3+ is a hard acid metal that can make strong bonds with hard base ligands such as carboxylic acids, amino nitrogens, hydroxamates and phenolates. One critical physiologic competitor for Ga3+ is transferrin protein which has a high-affinity binding constant (log K = 23.7) [22–23].
Chelating ligands such as DOTA, NOTA, HBED and NODAGA have been employed for making stable complexes. In addition, another chelator been used is TRAP. TRAP possesses phosphorous groups that hold very effective 68Ga. TRAP offers derivatization of the 3 phosphorous groups with other targeting molecules (Figure 2) [24–25].
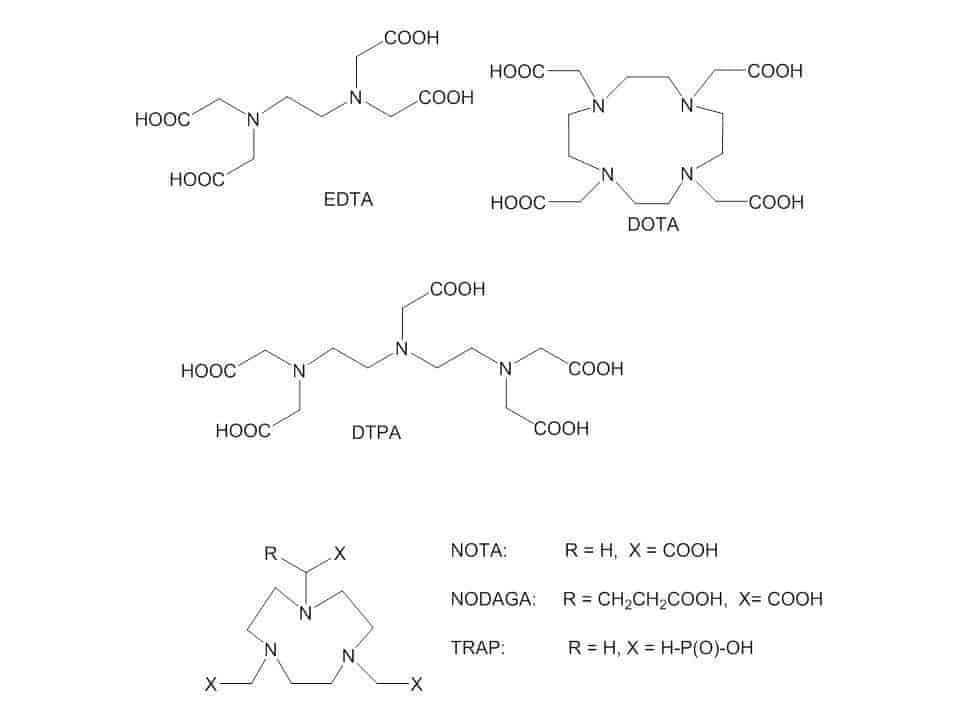
Figure 2. Examples of chelates of 68Ga.
Another advantage is the long half-life of the 68Ge/68Ga generator system which is 270 days. Consequently, there is the possibility of multiple elutions every day from this generator. Also, the cost of 68Ga is much reduced because there is no necessity of an on-site cyclotron. Schwaiger et al. hypothesized that 68Ga PET tracers can be used in well-established PET/CT nuclear medicine services at this current time [26].
For example, PET has been used for diagnosis and staging of gliomas and monitoring of treatment response. This focus is underscored because gliomas constitute around 45% of all brain tumours [27–28]. Currently, there exist a number of trials studying the efficacy of 68Ga-DOTATOC PET – (Ga-68 conjugate with a chelating ligand DOTA and small peptide) – when combined with MRI will differentiate embryonal tumours such as medulloblastoma and supratentorial primitive neuroectodermal tumour (PNET) from the low and high-grade gliomas [29–31]. Unfortunately, 68Ga PET imaging has not been extensively studied in β-amyloid yet [21].
Some of the advantages of using 68Ga in human PET imaging are the number of labelling potentialities and the possibility of freeze-dried kits alongside potentially a profitable market. However, there are still huge limitations for a broad-based human clinical usage such as pharmaceutical legislation of the generator to be classified for “human use” and demand for chelators that could be radiolabelled quickly at room temperature and at neutral pH [32].
2.1 Nanocarriers and antibodies radiolabelled with 68-gallium
Presently, nanocarriers such as liposomes, nanospheres and nanocrystals are usable as drug delivery systems. Our group is focusing on the development of specific-target nanoparticles radiolabelled with Gallium-68. The use of nanocarriers implies the awareness of their biodistribution, pharmacokinetic, and degradation property direction of these probes in vitro and in vivo [33]. There are few examples of nanoparticles using gallium-chemistry in literature [34]. Some of these nanoparticles were composed from organic polymers and iron oxide nanoparticles also utilising chelators such as NODAGA and NOTA for catching Gallium(III)-cation (Figure 3) [18–19,33–36].
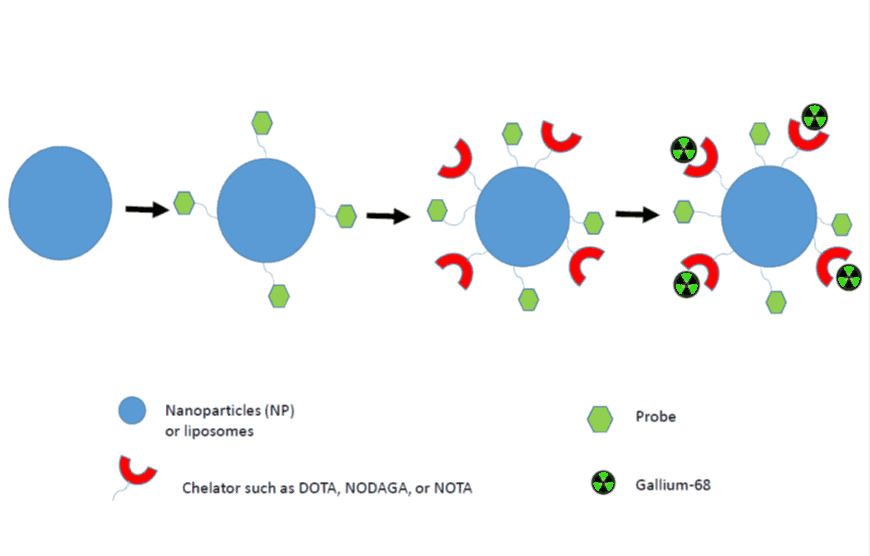
Figure 3. Schematic representation of 68Ga-labelled nanoparticle synthesis for in vivo imaging.
For example, Sing and Locatelli used NODAGA for the radiolabelling of gallium into nanogels and nanoparticles. However, there was a huge difference in the radiolabelling conditions and time procedures. The purification methods used after radiolabelling of Gallium have been solid phase extraction (SPE) and PD10-columns. In addition, Kim employed very moderate labelling conditions using pH 5.0-5.5 handling NOTA as a chelator. Stelter et al. did not employ any chelating agent; the authors used aminosilanes groups on the nanoparticles. Some of these nanoparticles were used as multimodal imaging agents for MRI and PET. It is important to mention that to our knowledge, there is no published work for a pre-radiolabelling approach using nanoparticles with Gallium-68 yet [18–19,33–36].
Conversely, monoclonal antibodies raised to target β-amyloid plaques and peptides fragments have been tested in patients with AD. Unfortunately, antibodies and peptides possess a limited uptake by the brain. Antibodies that bind to β-amyloid labelled with technetium-99 and iodine-125 using some modifications have been employed in imaging of amyloid in transgenic mice and brains of patients with AD [14].
3. Gallium-67 chemistry used in neuroinflammation imaging
Neuroinflammation is an entangled event that involves the responses of many cells such as neurons, macroglia, microglia and leukocytes. Neuroinflammation has been identified as the main component of many neurodegenerative conditions such as multiple sclerosis (MS), Alzheimer’s disease (AD), Parkinson’s disease (PD), narcolepsy and autism [37–38].
Gallium-67 citrate localizes inflammatory lesions by targeting leukocytes. Radiogallium is well known to bind tissue proteins like lactoferrin, transferrin and ferritin: these play a significant role in the inflammation process [39–40]. Gallium-67 citrate has also been used as a vehicle for blood-brain barrier scintigraphy [41]. However, gallium-67 has a number of flaws such as low specificity due to physiological bowel excretion and very strong accumulation in malignant cells. Gallium-67 as radiopharmaceutical has disadvantageous components such as high radiation-absorbed doses. The optimal imaging time of Gallium-67 is 72 h after injection [42].
Microglia plays a dominant aspect in the inflammation process too. For example, microglia triggers a fast response to brain injury with an accompanying expression of peripheral benzodiazepine receptor (PBR) [43]. So PBR, also called as the translocator protein TSPO (18 KDa) is a key target in the research of neuroinflammatory and brain disorders [44]. A carboximade ligand known as PK11195 binds tightly to PBR/TSPO. This ligand has been labelled with 11C for neuroimaging of the brain [45].
4. Current diagnostic agents for beta-amyloid imaging using gallium chemistry
Recently, scientists have made efforts in developing probes that display uptake and retention in AD patients’ brains for in vivo PET/SPECT. The scaffolds used are: chalcone, aurone, stilbene and oxadiazole, thioflavin-T, benzofuran and naphthalene. However, the most common radiolabelled probes in PET/SPECT are using 11C and 18F at this moment [46]. Gallium-68 is a promising radionuclide for in vivo imaging of β-amyloid plaques because it is easily produced by a generator.
In 1985, Friedland et al. worked with Ga-68 EDTA in dynamic PET studies. The authors made a quantitation of the passage of isotope from blood to brain in both healthy and AD patients. However, there were no significant differences between the groups [47].
Curcuminoids have demonstrated huge binding affinity for β-amyloid plaques. The theoretical and practical uses of Ga-curcuminoid – (phyto-compound and dietary spice usually named turmeric) – complexes have been tested by Asti et al. The authors described a synthesis route of curcuminoid complexes with a radiochemical yield higher than 95% at pH 5 with 5 min reaction time at 373 K. Asti reported that Ga-curcuminoid complexes strongly bind synthetic β-amyloid fibrils in preliminary data. However, quantitative inspection in vivo is still required for assessment of biodistribution of these complexes for a final outcome [48].
Chalcone is a set of biological molecules which possess an aromatic ketone and an enone core. Chauhan et al. selected a chalcone component that attaches stronger to β-amyloid plaques than neurofibrillary tangles and τ proteins existing in the AD brain. The authors employed diethylenetriaminepentaacetic acid (DTPA, Figure 2) as a chelator because it is demonstrated that these make complexes with trivalent metal ions. The 68Ga-DTPA conjugated two-chalcone structure was produced with a radiolabelled yield of 85%. An ex vivo biodistribution study established blood-brain barrier infiltration with an expeditious washout (30 minutes). These results could produce a new opportunity for future radiopharmaceuticals in base of chalcone derivatives conjugate with 68Ga [21].
5. Future directions
Many scientists from different fields have been examined biomarkers for an optimal biomarker in AD. By paying attention to the entanglement of the AD disease progress, it is suggested that it is very unlikely that only one diagnostic or biomarker is the solution for AD identification or treatment. There is a further need to standardize the biomarkers, as well as new techniques for the assessment of surrogate biomarkers [3].
The new generation of tracers and ligands such as bifunctional chelators that could be conjugated to either one or multiples peptides or vectors are needed to contribute to the clinical use of 68Ge/68Ga generators. Furthermore, an equivalent 68Ga radiopharmaceutical is to be used in molecular imaging. One interesting aspect of 68Ga-PET/CT imaging is its application in therapy. Considering some bifunctional chelators that coordinate with Ga(III) will possess the same coordination chemistry as 90Y and 177Lu there is a potential for a theranostic approach [49].
Acknowledgements
This project was funded by the Academy of Finland (project 260316).
Conflict of interest
The authors confirm that this article content has no conflict of interest.
References
Key References: 14, 15, 21, 24, 48
- Cheng Y, Ono M, Kimura H, Ueda M, Saji H. Technetium-99m labeled pyridyl benzofuran derivatives as single photon emission computed tomography imaging probes for β-amyloid plaques in Alzheimer’s brains. J Med Chem. 2012; 55(5): 2279-2286.[Reference Source] [PubMed Abstract]
- Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer’s disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement. 2012; 8: S1-S68.[PMC] [PubMed Abstract]
- Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement. 2005; 1(1): 55-66.[PMC] [PubMed Abstract]
- Small G, Kepe V, Ercoli L, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006; 355(25): 2652-2663.[Cross Ref] [PubMed Abstract]
- Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta. 2012; 1822(3): 370-379.[Reference Source] [PubMed Abstract]
- Villemagne VL, Fodero-Tavoletti MT, Pike KE, Cappai R, Masters CL, Rowe CC. The ART of loss: Aβ Imaging in the evaluation of Alzheimer’s disease and other dementias. Mol Neurobiol. 2008; 38(1): 1-15.[Cross Ref] [PubMed Abstract]
- Gross S, Piwnica-Worms D. Molecular imaging strategies for drug discovery and development. Curr Opin Chem Biol. 2006; 10(4): 334-342.[Cross Ref] [PubMed Abstract]
- Berndt M, Brockschnieder D, Heinrich T, et al. Diagnostic agents for amyloid beta imaging. Patent WO2011141515A1 Accessed February 12, 2015.[Reference Source]
- Joshi S, Ornstein E, Bruce JN. Targeting the brain. Neurocrit Care. 2007; 6(3): 200-212.[Cross Ref] [PubMed Abstract]
- Sahoo S, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003; 8(4): 1112-1120.[CrossRef] [PubMed]
- Pardridge W. Drug delivery to the Brain. J Cereb Blood flow Metab. 1997; 17:713-731. [PubMed]
- Basu S, Kwee T, Surti S, Akin EA, Yoo D, Alavi A. Fundamentals of PET and PET/CT imaging. Ann NY Acad Sci. 2011; 1228: 1-18.[Cross Ref] [PubMed Abstract]
- Barrios-Lopez B, Bergström K. Radiolabeled sugars used for PET and SPECT imaging: Mini-Review. Curr Radiopharm. 2015 May 24. [Epub ahead of print][Reference Source] [PubMed Abstract]
- Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004; 3(9): 519-527.[Reference Source] [PubMed Abstract]
- Barrios-Lopez B, Raki M, Bergström K. Radiolabeled peptides for Alzheimer’s diagnostic imaging: mini-Review. Curr Radiopharm. 2013; 6(4): 181-191.[Reference Source] [PubMed Abstract]
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004; 55(3): 306-319.[Reference Source] [PubMed Abstract]
- Kung HF. The beta-amyloid hypothesis in Alzheimer’s disease: seeing is believing. ACS Med. Chem. Lett. 2012; 3(4): 265-267.[PMC] [PubMed Abstract]
- Stelter L, Pinkernelle JG, Michel R, et al. Modification of aminosilanized superparamagnetic nanoparticles: feasibility of multimodal detection using 3T MRI, small animal PET, and fluorescence imaging. Mol Imaging Biol. 2010; 12(1): 25-34.[Cross Ref] [PubMed Abstract]
- Kim SM, Chae MK, Yim MS, et al. Hybrid PET/MR imaging of tumors using an oleanolic acid-conjugated nanoparticle. Biomaterials. 2013; 34(33): 8114-8121.[Cross Ref] [PubMed Abstract]
- Elsinga PH. Present and future of PET-radiopharmaceuticals. Nucl Med Rev. 2012; 15: C13-C16.[Reference Source]
- Chauhan K, Datta A, Adhikari A, Chuttani K, Kumar Singh A, Mishra AK. 68 Ga Based probe for Alzheimer’s disease: synthesis and preclinical evaluation of homodimeric chalcone in β-Amyloid imaging. Org Biomol Chem. 2014; 12(37): 7328-7337.[Reference Source] [PubMed Abstract]
- Maecke HR, Hofmann M, Haberkorn U. 68Ga-labeled peptides in tumor imaging. J Nucl Med. 2005; 46:172S-178S.[Reference Source] [PubMed Abstract]
- Primetech Corporation. Ge-68/Ga-68 generator. Easy and direct labeling of PET tracers.
- Notni J, Šimeček J, Hermann P, Wester HJ. TRAP, a Powerful and versatile framework for gallium-68 radiopharmaceuticals. Chem Eur J. 2011; 17(52): 14718-14722.[Cross Ref] [PubMed Abstract]
- Berry DJ, Ma Y, Ballinger JR, et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: tris(hydroxypyridinone) ligands. Chem Commun. 2011; 47(45): 7068-7070.[Cross Ref] [PubMed Abstract]
- Schwaiger M, Wester H-J. How many PET tracers do we need? J Nucl Med. 2011; 52: 36S-41S.[Cross Ref] [PubMed Abstract]
- Bansal A, Wong TZ, DeGrado TR (2011). PET imaging of gliomas. Advances in the biology, imaging and therapies for glioblastoma, Clark C. Chen (Ed.). ISBN: 978-953-307-284-5, InTech.
- Benard F, Romsa J, Hustinx R. Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med. 2003; 33(2): 148-162.[Cross Ref] [PubMed Abstract]
- Clinical Trials: Efficacy of 68Ga-DOTATOC positron emission tomography (PET) CT in children and young adults with brain tumors.
- Hofmann M, Maecke H, Börner R, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001; 28(12): 1751-1757.[Cross Ref] [PubMed Abstract]
- Poeppel T, Binsel I, Petersenn S, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011; 52(12):1864-1870.[Cross Ref] [PubMed Abstract]
- Vorster M, Maes A, van deWiele C, Sathekge M. Gallium-68: a systematic review of its nononcological applications. Nucl Med Commun. 2013; 34(9): 834-854.[Cross Ref] [PubMed Abstract]
- Singh S, Bingöl B, Morgenroth A, Mottaghy FM, Möller M, Schmaljohann J. Radiolabeled nanogels for nuclear molecular imaging. Macromol Rapid Commun. 2013; 34(7): 562-567.[Cross Ref] [PubMed Abstract]
- Stockhofe K, Postema J, Schieferstein H, Ross TL. Radiolabeling of nanoparticles and polymers for PET imaging. Pharmaceuticals (Basel). 2014; 7(4): 392-418.[Cross Ref] [PubMed Abstract]
- Locatelli E, gil L, Israel LL, et al. Biocompatible nanocomposite for PET/MRI hybrid imaging. Int J Nanomedicine. 2012; 7: 6021-6033.[Reference Source] [PubMed Abstract]
- Cartier R, Kaufner L, Paulke BR, et al. Latex nanoparticles for multimodal imaging and detection in vivo. Nanotechnology. 2007; 195102. [Cross Ref] [Reference Source]
- Venneti S, Lopresti B, Wiley CA. The peripheral benzodiazepine receptor (Translocator protien 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006; 80(6): 308-322.[PMC] [PubMed Abstract]
- Venneti S, Lopresti B, Wang G, et al. A comparison of the high-affinity peripheral benzodiazepine receptor ligands DAA1106 and (R)-PK11195 in rat models of neuroinflammation: implications for PET imaging of microglial activation. J Neurochem. 2007; 102(6): 2118-2131.[Cross Ref] [PubMed Abstract]
- Dhawan VM, Szjklas JJ, Spencer RP. Localization of Ga-67 in inflammations in the absence of circulation polymorphonuclear leukocytes. J Nucl Med. 1978; 19(3): 292-294.[Reference Source] [PubMed Abstract]
- Signore A, Glaudemans AW. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann Nucl Med. 2011; 25(10): 681-700.[Cross Ref] [PubMed Abstract]
- Ell PJ. Nuclear Medicine. Postgrad Med J. 1992; 68(796): 82-105.[Cross Ref] [PubMed Abstract]
- Sharma V, Prior JL, Belinsky MG, Kruh GD, Piwnica-Worms D. Characterization of a 67Ga/68 Ga radiopharmaceutical for SPECT and PET of MDR1 P-glycoprotein transport activity in vivo: validation in multidrug-resistant tumors and at the blood-brain barrier. J Nucl Med. 2005; 46(2): 354-364.[Reference Source] [PubMed Abstract]
- Debruyne JC, Versijpt J, Van Laere KJ, et al. PET visualization of microglia in multiple sclerosis patients using [11-C]-PK11195 PET. Eur J Neurol. 2003; 10(3): 257-264.[Reference Source] [PubMed Abstract]
- Schweitzer PJ, Fallon BA, Mann JJ, Kumar JS. PET tracers for the peripheral benzodiazepine receptor and uses thereof. Drug Discov Today. 2010; 15(21-22): 933-942.[Cross Ref] [PubMed Abstract]
- Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11-C]-PK11195 PET. Mov Disord. 2007; 22(13): 1852-1856.[Reference Source] [PubMed Abstract]
- Eckroat TJ, Mayhoub AS, Garneau-Tsodikova S. Amyloid-β probes: Review of structure- activity and brain-kinetics relationships. Beilstein J Org Chem. 2013; 9:1012-1044.[CrossRef] [PubMed]
- Friedland RP, Jagust WJ, Budinger TF, Yano Y, Huesman RH, Knittel B. Dynamic PET studies of blood-brain barrier permeability in Alzheimer’s disease. J Nucl Med; 1985. Conference 32: Annual Meeting of the Society of Nuclear Medicine.[Reference Source]
- Asti M, Ferrari E, Croci S, et al. Synthesis and characterization of 68 Ga-labeled curcumin and curcuminoid complexes as potential radiotracers for imaging of cancer and Alzheimer’s disease. Inorg Chem. 2014; 53(10): 4922-4933.[Reference Source] [PubMed Abstract]
- Rösch F. Past, present and future of 68Ge/68Ga generators. Appl Radiat Isotopes. 2013; 76: 24-30. [Reference Source] [PubMed Abstract]
Article information
Corresponding author: Kim Bergström.
Copyright: © 2015 Barrios-Lopez B et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are cited.
How to cite: Barrios-Lopez B, Airaksinen A, Bergström K. Gallium-68 radiotracers for Alzheimer’s plaque imaging. J. Diagn. Imaging Ther. 2015; 2(2): 50-65 (https://dx.doi.org/10.17229/jdit.2015-0930-019).
Article history: Received 21 August 2015; Revised 23 September 2015; Accepted: 29 September 2015; Published online 30 September 2015.
Archive link: JDIT-2015-0930-019
You Are Here: Home »
Tags: Alzheimer’s disease, Gallium-68 Radiotracers, PET Amyloid Imaging