RESEARCH ARTICLE
Alessandro Sperandeo1, Umberto Ficola1, Natale Quartuccio 2, Sean L. Kitson 3, Luigi Mansi 4,*, Angelina Cistaro 2
1 Nuclear Medicine Oncologic Dept, La Maddalena Hospital, Palerm, Italy
2 Positron Emission Tomography Centre IRMET S.p.A., Turin, Italy
3 Department of Biocatalysis and Isotope Chemistry, Almac, 20 Seagoe Industrial Estate, Craigavon, BT63 5QD, United Kingdom
4 Medicina Nucleare, Seconda Università di Napoli, Piazza Miraglia, 80138, Napoli, Italy
Abstract
The automated radiosynthesis of [18F]fluorocholine was carried out using a modified reactor design in the GE TracerLab FX(FDG) module. This PET imaging tracer was synthesized in two steps and involved the generation of nucleophilic [18F]fluoride using Kryptofix 2.2.2 technology. The first step was the synthesis of [18F]fluorobromomethane by the reaction of dibromomethane with [18F]fluoride mediated by Kryptofix 2.2.2. Then the substrate N,N-dimethylaminoethanol was N-alkylated with [18F]fluorobromomethane to give the [18F]fluorocholine as the bromide salt. Purification of the salt using Sep-Pak silica gel gave [18F]fluorocholine in a radiochemical purity ≥99% area. This new reactor design in the TracerLab module produced high radiochemical purity and reproducible yields of [18F]fluorocholine. Furthermore, this automated approach could be implemented for routine PET imaging of oncological disease states in the Nuclear Medicine clinical setting.
Keywords: automated radiosynthesis; positron emission tomography; fluorine radioisotopes; choline; 2-deoxy-2-[18F]fluoro-D-glucose; radiopharmaceuticals.
1. Introduction
Choline is a quaternary ammonium salt and is an important constituent of various biological molecules [1]. It is used as a biosynthetic precursor in two processes such as in the synthesis of phospholipids and acetylcholine [2]. Choline is used for the synthesis of a particular phospholipid called phosphatidylcholine (lecithin) [3]. The metabolic pathway consists of three different steps. In the first step, the hydroxyl moiety on choline undergoes phosphorylation to choline-phosphate. This step is facilitated by adenosine triphosphate (ATP). In the penultimate step choline-phosphate reacts with CTP (choline-phosphate cytidylyltransferase) releasing pyrophosphate and forming the cytidine-5′-diphosphocholine (CDP-choline). The final step involves the conversion of CDP-choline to phosphatidylcholine [4].
Some tumour cells are characterized by an increased uptake of choline and an over-expression of the genes encoding the choline kinase enzyme. This leads to high levels of phosphocholine available for the formation of phosphatidylcholine, whose biosynthesis is particularly increased in the tumour cells [5].
Several studies have shown that cancer cells, especially in prostate cancer, have high concentrations of choline and decreased citrate levels [6]. The use of PET radiolabelled choline analogues for in vivo imaging, exploits these metabolic abnormalities to enabling detection of cancerous cells [7]. Choline was also found effective as a diagnostic tool in low-grade brain tumours, as precursor of the neurotransmitter acetylcholine [8]. Choline is taken up into the malignant cells and converted into phosphorylcholine, remaining trapped inside the cells. The next step is the synthesis of phosphatidylcholine which represents a key component of cell membranes [9].
Recent reports have shown that the use of the radionuclide fluorine-18, instead of the routinely used carbon-11, could be a valuable alternative for radiolabelling choline [10]. In addition, a further benefit of this radionuclide is dependent on its longer half-life, approximately five times greater than carbon-11 (t1/2 of carbon-11 = 20.4 min and t1/2 of fluorine-18 = 109.8 min). Therefore fluorinated radiocompounds allow to perform a greater number of clinical examinations per imaging session, and also permitting transportation of the radiopharmaceutical to other PET imaging centres, not having a cyclotron [11].
The aim of our work is to implement, for a routine clinical use, the investigational diagnostic PET radiopharmaceutical [18F]fluorocholine, known as [18F]FCH, on a commercial synthesis module, i.e. the TracerlabFXFDG [12].
2. Materials and methods
(Note: For all the major products, include company name and registered office of the factory, when mentioned for the first time).
All chemicals and reagents were purchased from Sigma-Aldrich and included dibromomethane, N,N-dimethylaminoethanol, acetonitrile, ethanol, Kryptofix 2.2.2, potassium carbonate. The Sep-Pak cartridges were obtained from Waters and the reference standard fluorocholine was from ABX.
2.1. Production of 18F
The application of the Cyclotron 18/9 IBA, equipped with a niobium target was used in the production of fluorine-18, by the irradiation of enriched water containing oxygen-18 > 97%.
2.2. Instrumentation
The radioactivity of the tracer [18F]FCH was measured using a calibrated Capintec CRC-15 PET instrument. The HPLC analysis of [18F]FCH2Br was performed with the GP50 pump (Dionex), UV-Visible Detector (UVD 170U, Dionex), a reversed phase HPLC column (Acclaim 120 C18, 4 mm x 250 mm, Dionex) and a radioactivity detector (Bioscan Flow Counter). The solvent eluents water/acetonitrile (30/70) were used with a flow rate of 3 mL/min.
The quality control of [18F]FCH was performed with a GP50 pump (Dionex), a conductometric detector, a suppressor CRSR 300; a column (IonPac CS12A 4 mm x 250 mm, Dionex), a radioactivity detector (Bioscan Flow Counter) and a Mobil phase 20 mM MSA, working at a flow of 1 mL/min and using a current of 59 mA.
2.3. Gas-chromatography analysis
The analysis of residual solvents and DMAE, were performed using the gas chromatography system GC2010AF. The gas chromatograph (Shimadzu), was equipped with two independent chromatography lines, coupled to FID detectors. The analysis of residual solvents (acetonitrile, acetone and ethanol), was performed using the column Supelcowax 10. The analysis of DMAE, was conducted using the Rtx-5 Amine column.
2.4. Synthesis of [18F]fluorocholine
The synthesis of [18F]FCH has been made in a two-steps process (Scheme 1) by alkylation on a solid support (Sep-Pak tC18) of DMAE by [18F]FCH2Br. This is the product of nucleophilic substitution of CH2Br2 by the [18F]fluoride. The 18F (no-carrier-added) products were transferred to the hot cells using synthetic PTFE lines and separated using QMA (Quaternary methyl ammonium). The isotope is eluted from the QMA with a solution of water/acetonitrile containing 2.5 mg of K2CO3 and 20 mg of Krytofix 2.2.2. The solution evaporated after 3 minutes at 110ºC and the residue was triturated with two successive additions of anhydrous acetonitrile (2 x 1 mL). Following this step, a 10% solution of dibromomethane in anhydrous acetonitrile was added, by maintaining a temperature of 95ºC over 4 minutes.
During this phase, the reactor out-let was connected to a Sep-Pak Silica cartridge and opened to allow removal of the volatile component that had been formed. After the formation of [18F]FCH2Br, a stream of helium, at a flow rate of 30 mL/min, passed through the Sep-Pak silica cartridge separating the [18F]FCH2Br. This was eluted between the third and tenth minute, through the Sep–Pak tC18, containing 300 μL of DMAE (Figure 1).
Purification of [18F]FCH was made on a cation exchange cartridge (Accell CM), connected in series to the Sep–Pak tC18. The eluents used in the purification process were 10 mL of ethanol and 10 mL of water. The final elution was performed using 3 mL of saline. The finished product was sterilized by filtration, by using a 0.22 micron filter and gave a radiochemical purity ≥ 99% area.
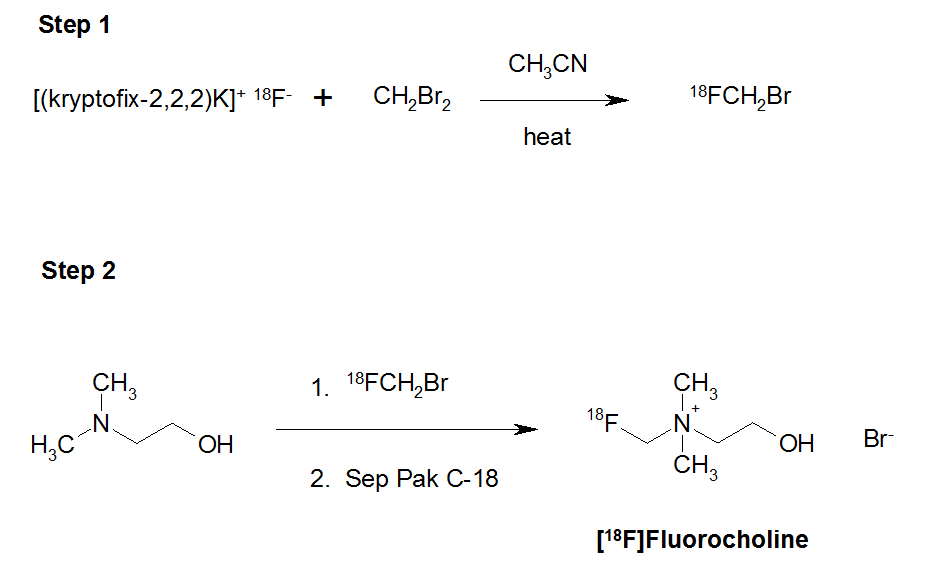
Scheme 1. Synthesis of [18F]fluorocholine.
In order to identify the synthesized molecule, ‘cold’ synthesis with [19F]KF, under the same conditions used for the synthesis of the radiopharmaceutical were conducted. The obtained product was analyzed by HPLC and Nuclear Magnetic Resonance (NMR). The results were compared with a commercial standard of [19F]FCH (Table 1).
Table 1. Nuclear Magnetic Resonance analysis results (1H-NMR (300 MHz; CD3OD).
3. Results
To optimize the production of the volatile radioactive component [18F]FCH2Br it was eluted from the Sep-Pak silica gel cartridge, the compound was trapped in 500 μL of N,N-dimethylformamide at 0 ºC for 1 minute.
The different elution rates from the Sep-Pak silica gel cartridge were analyzed using HPLC system 1. The retention time of [18F]FCH2Br and CH2Br2 was 2.2 and 4.0 minutes, respectively. The radio-chromatograms indicated the presence of impurities for the first three aliquots of N,N-dimethylformamide that corresponded to the first three minutes of elution (Figure 1).
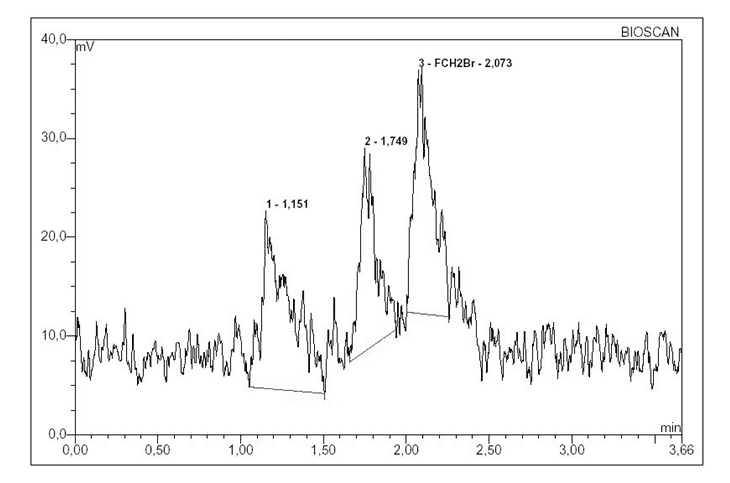
Figure 1. Radio-chromatogram showing presence of impurity in the first three aliquots.
The analysis of subsequent rates of N,N-dimethylformamide observed that [18F]FCH2Br had a radiochemical purity ≥ 98% area (Figure 2). The [18F]FCH2Br was separated between 3-12 minutes.
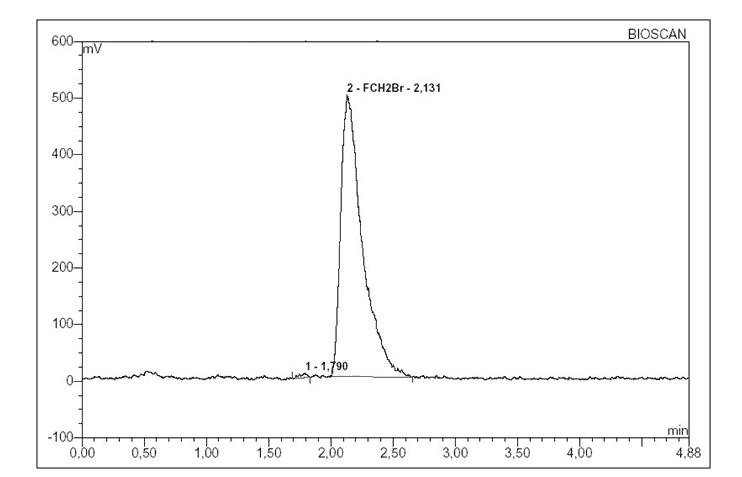
Figure 2. Radio–chromatogram showing purity of [18F]FCH2Br.
The radiochemical purity of [18F]FCH, determined by using the second HPLC system was ≥99% area. The final product characteristics are summarized in Table 2.
Table 2. Characteristics of [18F]fluorocholine PET imaging tracer.
The stability of the radiopharmaceutical [18F]FCH was determined by analyzing a sample at a concentration of 75 mCi/mL. The tests performed on [18F]FCH included the radiochemical purity and pH at the time intervals of 2, 5 and 10 hours. At each time interval, an aliquot of 500 μL was withdrawn from the main vial. The analysis of the stability data, indicated that the radiochemical purity of [18F]FCH after 10 hours of production remained within acceptable limits, to be utilized in medical imaging. A typical radiochromatogram for the analysis of [18F]FCH is shown in Figure 3.
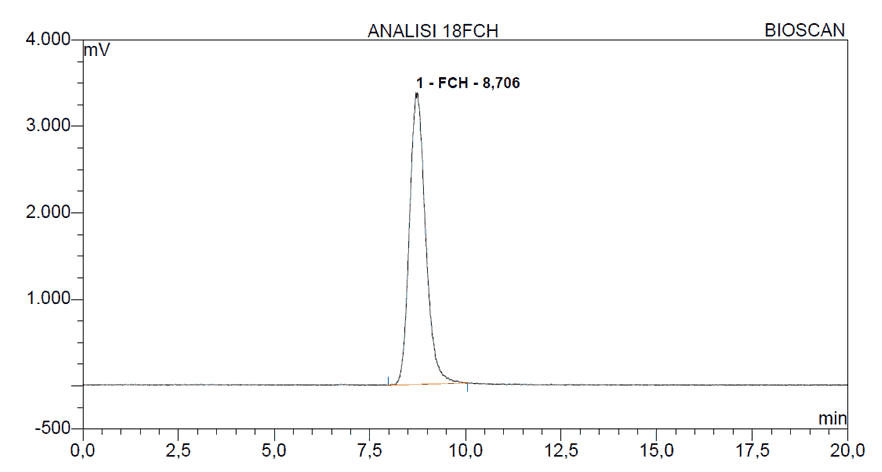
Figure 3. HPLC radio-chromatogram of [18F]FCH.
4. Discussion
In 1976, Abass Alavi was the first physician to administer the glucose analogue, 2-deoxy-2-[18F]fluoro-D-glucose known as [18F]FDG to a patient and obtained whole body and brain tomographic images [13]. [18F]FDG contains the positron-emitting short-lived radionuclide fluorine-18 which has been substituted for a hydroxyl moiety at position 2’ in the glucose molecule [14]. Currently, this radiopharmaceutical is widely utilized in Nuclear Medicine with major use in positron emission tomography (PET) imaging in the application, management and detection of oncological disease states [15]. In addition,[18F]FDG is also used as a standard radiotracer for PET neuro-imaging and diagnostics of cardiac and inflammatory diseases [16].
The role of [18F]FDG in oncology is dependent on its affinity to accumulate inside the tumour cell where higher glucose metabolism takes place compared to normal surrounding cells [17]. However, not all tumours demonstrate significant increased metabolism of [18F]FDG and this in principle is mainly true for prostate cancer, neuroendocrine tumours and hepatic malignancies [18]. For this reason, other PET radiotracers have been developed to target alternative biological processes of cancer biology [19]. One particular radiotracer of interest is choline radiolabelled with fluorine-18 or carbon-11 for the use of PET imaging [20].
The aim of our work was to implement, for routine clinical use, the investigational diagnostic PET radiopharmaceutical [18F]fluorocholine, known as [18F]FCH, on a commercial synthesis module, i.e. the TracerlabFXFDG [12]. The synthesis of [18F]FCH was carried out in two steps (Scheme 1). The first reaction step involved the generation of [18F]fluorobromomethane, [18F]FCH2Br. This was produced from the mono-nucleophilic substitution of dibromomethane using [18F]fluoride anion generated from the thermal decomposition of the kryptofix-2.2.2.K[18F-] salt in anhydrous acetonitrile.
In step 2, the [18F]fluorobromomethane was used to alkylate the tertiary amine, N,N-dimethylaminoethanol to give a crude bromide salt of [18F]FCH. This material was purified and isolated using silica gel Sep-Pak cartridges.
The automated synthesis module TracerLab is dedicated to the fast and reliable production of [18F]FDG. The most difficult change made was the design of the reactor vessel. The default module is equipped with a glass-carbon reactor vessel with a maximum capacity volume of 22 mL. In the first step of the synthesis of [18F]FCH2Br a significant reduction in the radioactivity was observed, probably due to the excessive volume of the reactor allowing a difficult trapping of the [18F]FCH2Br in the gas phase.
To circumvent these reactor problems, a scaled ‘test’ reactor in Pyrex glass was made to enable reflux, compatible with the existing heating system, within the TracerLab module. Initially, there were some difficulties in determining the elution profile of [18F]FCH2Br because a radio-detector was not present at the outlet of the silica cartridges.
To maintain repeatable elution times for the production of [18F]FCH2Br, it was necessary to control the helium flow before each synthesis run. Therefore, several tests were performed in order to reduce the residual N,N-dimethylaminoethanol (DMAE).
It was necessary to condition the Sep-Pak Accell CM cartridges with aqueous hydrochloric acid (0.5 M); the residual DMAE formed was between 300 and 400 μg/mL. When the first test of conditioning was performed with water, the residual DMAE was between 40 and 50 μg/mL.
Therefore, using unconditioned Sep-Pak Accell CM cartridges, a final DMAE residual less than 15 μg/mL was obtained. Moreover, using an unconditioned Sep-Pak Accell CM cartridge causes a reduction in radiochemical yield, because [18F]fluorocholine was not completely retained by the Sep-Pak Accell CM cartridge.
5. Conclusion
The implementation of [18F]fluorocholine synthesis on a modified commercial synthesis module, i.e. the Tracerlab has been proved to be safe and gave high, reproducible radiochemical yields. This approach to the synthesis of [18F]fluorocholine will play a key role in the management and diagnosis of cancer patients.
Conflict of interest
The authors confirm that this article content has no conflicts of interest.
References
Key References: 1, 5, 6, 10, 12, 14, 16, 20
- Blusztan JK, Liscovitch M, Richardson UI. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. PNAS. 1987; 84(15): 5474-5477. [CrossRef] [PubMed Abstract]
- Jope RS, Jenden DJ. Choline and phospholipid metabolism and the synthesis of acetylcholine in rat brain. J Neurosci Res. 1979; 4(1): 69-82. [CrossRef] [PubMed Abstract]
- Blusztajn JK, Liscovitch M, Mauron C, Richardson UI, Wurtman RJ. Phosphatidylcholine as a precursor of choline for acetylcholine synthesis. J Neural Transm Suppl. 1987; 24: 247-259. [PubMed Abstract]
- Glunde K, Jacobs MA, Bhujwalla ZM. Choline metabolism in cancer: implications for diagnosis and therapy. Expert Review of Molecular Diagnostics. 2006; 6(6): 821-829. [CrossRef] [PubMed Abstract]
- Plathow C, Weber WA. Tumor cell metabolism imaging. J Nucl Med. 2008; 49: 43S-63S. [CrossRef] [PubMed Abstract]
- Awward HM, Geisel J, Obeid R. The role of choline in prostate cancer. Clin Biochem. 2012; 45(18): 1548-1553. [PubMed Abstract]
- Witney TH, Alam IS, Turton DR, Smith G, Carroll L, Brickute D, et al. Evaluation of deuterated 18F- and 11C-labeled choline analogs for cancer detection by positron emission tomography. Clin Cancer Res. 2012; 18(4): 1-10. [CrossRef] [PubMed Abstract]
- Hara T, Kosaka N, Shinoura N, Kondo T. PET imaging of brain tumor with [methyl- 11C]choline. J Nucl Med. 1997; 38(6): 842-847. [PubMed Abstract]
- Gibellini F, Smith TK. The Kennedy pathway-De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. 2010; 62(6): 414-428. [CrossRef] [PubMed Abstract]
- Schmid DT, John H, Zweife, R, Cservenyak T, Westera G, Goerres GW, et al. Fluorocholine PET/CT in Patients with Prostate Cancer: Initial Experience. Radiology. 2005; 235(2): 623-628. [PubMed Abstract]
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of Fluorine-18 Positron Emission Tomography/Computed Tomography for Identification of Cardiovascular Implantable Electronic Device Infections. J Am Coll Cardiol. 2012; 59(18): 1616-1625. [CrossRef] [PubMed Abstract]
- Shao X, Hoareau R, Hockley BG, Tluczek LJM, Henderson BD, Padgett HC, Scott PJH. Highlighting the Versatility of the Tracerlab Synthesis Modules. Part 1: Fully Automated Production of [F]Labelled Radiopharmaceuticals using a Tracerlab FX(FN). J Label Compd and Radiopharm. 2011; 54(6): 292-307. [PubMed Abstract] [PMC Free Article]
- Wagner HN. A personal History of Nuclear Medicine, Springer Ed; London, 2006. [Reference Source]
- Ido T, Wan C-N, Casella V, Fowler JS, Wolf A P, Reivich M, Kuhl D E. Labeled 2-deoxy-D-glucose analogs. 18F-labeled 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-fluoro-D-mannose and 14C-2-deoxy-2-fluoro-D-glucose. J Label Compd Radiopharm. 1978; 14:175–183. [CrossRef]
- Delbeke D. Oncological applications of FDG-PET imaging. J Nucl Med. 1999; 40: 1706-1715. [PubMed Abstract]
- Kitson SL, Cuccurullo V, Ciarmiello A, Salvo D, Mansi L. Clinical Applications of Positron Emission Tomography (PET) Imaging in Medicine: Oncology, Brain Diseases and Cardiology. Curr Radiopharm. 2009; 2: 224-253. [CrossRef]
- Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Mazière B. FDG accumulation and tumor biology. Nucl Med Biol. 1998; 25: 317-322. [CrossRef] [PubMed Abstract]
- Caroli P, Nanni C, Rubello D, Alavi A, Fanti S. Non-FDG PET in the practice of oncology. Indian J Cancer. 2010; 47, 120-125. [CrossRef] [PubMed Abstract]
- Chen K, Chen X. Positron emission tomography imaging of cancer biology: current status and future prospects. Semin Oncol. 2011; 8: 70-86. [CrossRef] [PubMed Abstract]
- Treglia G, Giovannini E, Di Franco, D, Calcagni ML, Rufini V, Picchio M, Giordano A. The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: a systematic review. Ann Nucl Med. 2012; 26: 451-461. [CrossRef] [PubMed Abstract]
Article information
Corresponding author: Luigi Mansi.
Copyright: © 2014 Mansi L et al.
How to Cite: Sperandeo A, Ficola U, Quartuccio N, Kitson SL, Mansi L, Cistaro A. Automated synthesis of [18F]fluorocholine using a modified GE tracerlab module. J. Diagn. Imaging Ther. 2014; 1(1):49-58 ( https://dx.doi.org/10.17229/jdit.2014-0921-003).
Article history: Received 02 September 2014; Revised 19 September 2014; Accepted 19 September 2014; Published online 21 September 2014.
Article link: JDIT-2014-0921-003
You are here: home »
