Radiotherapeutics
The landscape of medical radiotherapeutics is rapidly evolving, showcasing a multitude of innovative treatments that target specific cancers and related symptoms with unprecedented precision. Utilising various radionuclides, these therapies represent a new frontier in the fight against cancer, offering hope for more effective and personalised treatment options.
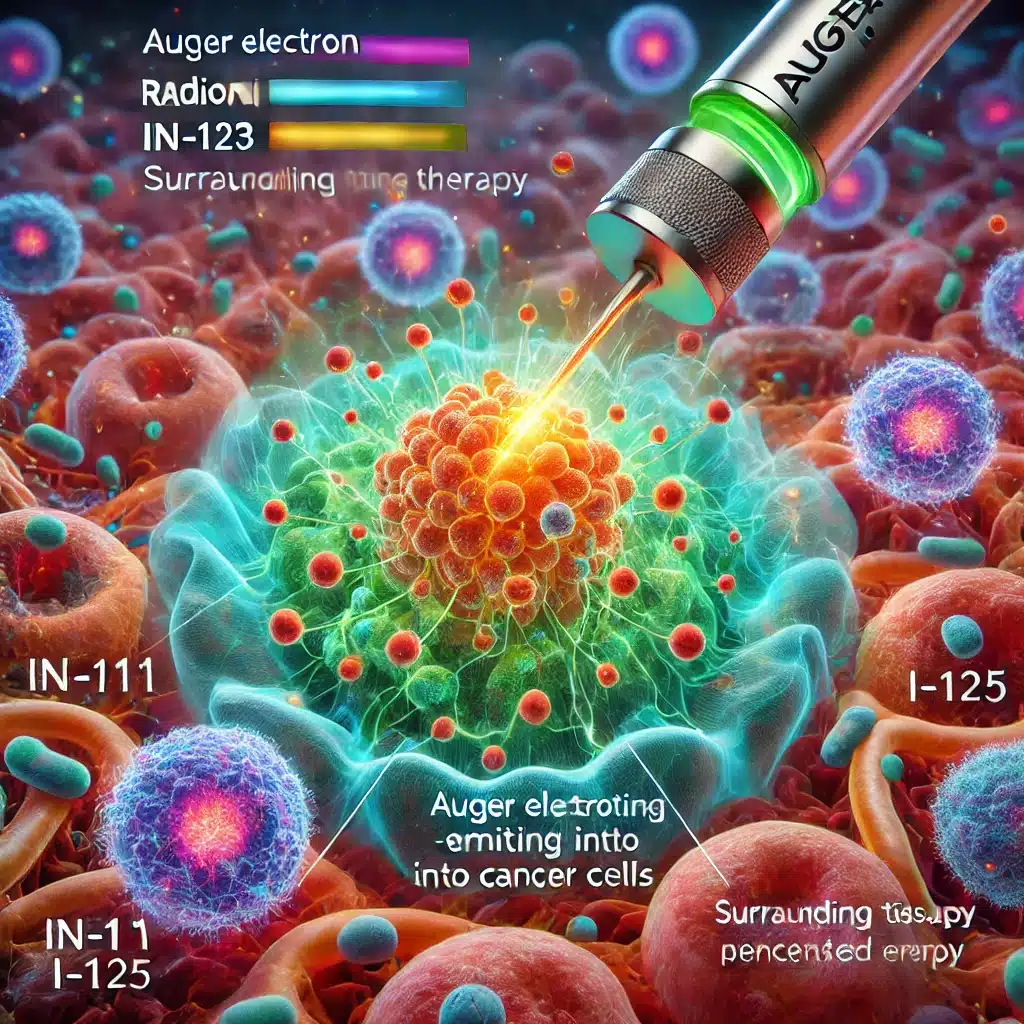
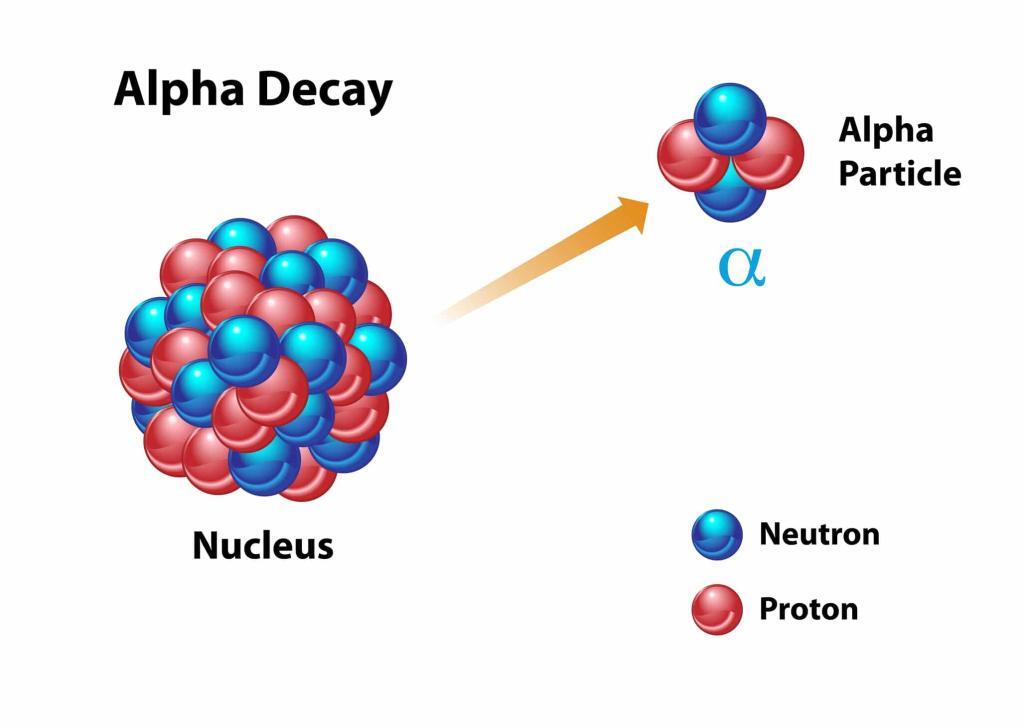
Central to this advancement is the use of Actinium-225 (Ac-225), an alpha-emitting radionuclide, in a range of promising therapies. Alpha particles, with their high energy and short range, are effective in destroying cancer cells while minimising collateral damage to surrounding healthy tissues.
For instance, Ac-225-DOTA-SP targets Substance P receptors in glioblastoma, a notably aggressive brain tumour, delivering alpha radiation directly to the tumour site. This targeted approach is echoed in Ac-225-DOTA-YS5 and Ac-225-FPI-2265, both aimed at treating prostate cancer through different mechanisms. Ac-225-DOTA-YS5 targets CD46 using monoclonal antibody IgG1, whereas Ac-225-FPI-2265 focuses on the Prostate-Specific Membrane Antigen (PSMA) with PSMA-I&T.
The targeting of neuroendocrine tumours (NETs) has also seen significant developments with agents like Ac-225-DOTATOC and Ac-225-RYZ101, which focus on somatostatin receptors, delivering alpha radiation precisely to tumour cells. Further, Ac-225-DOTAZOL for bone pain palliation in patients with bone metastases exemplifies the versatility of radiotherapeutics, directly targeting bone tissues to provide pain relief.
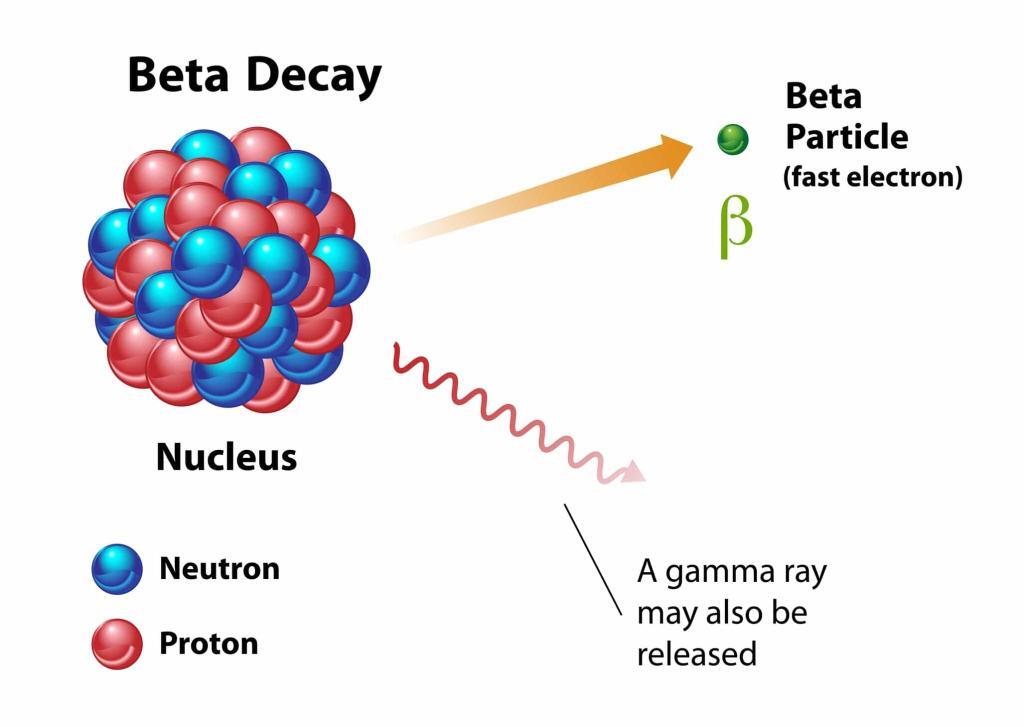
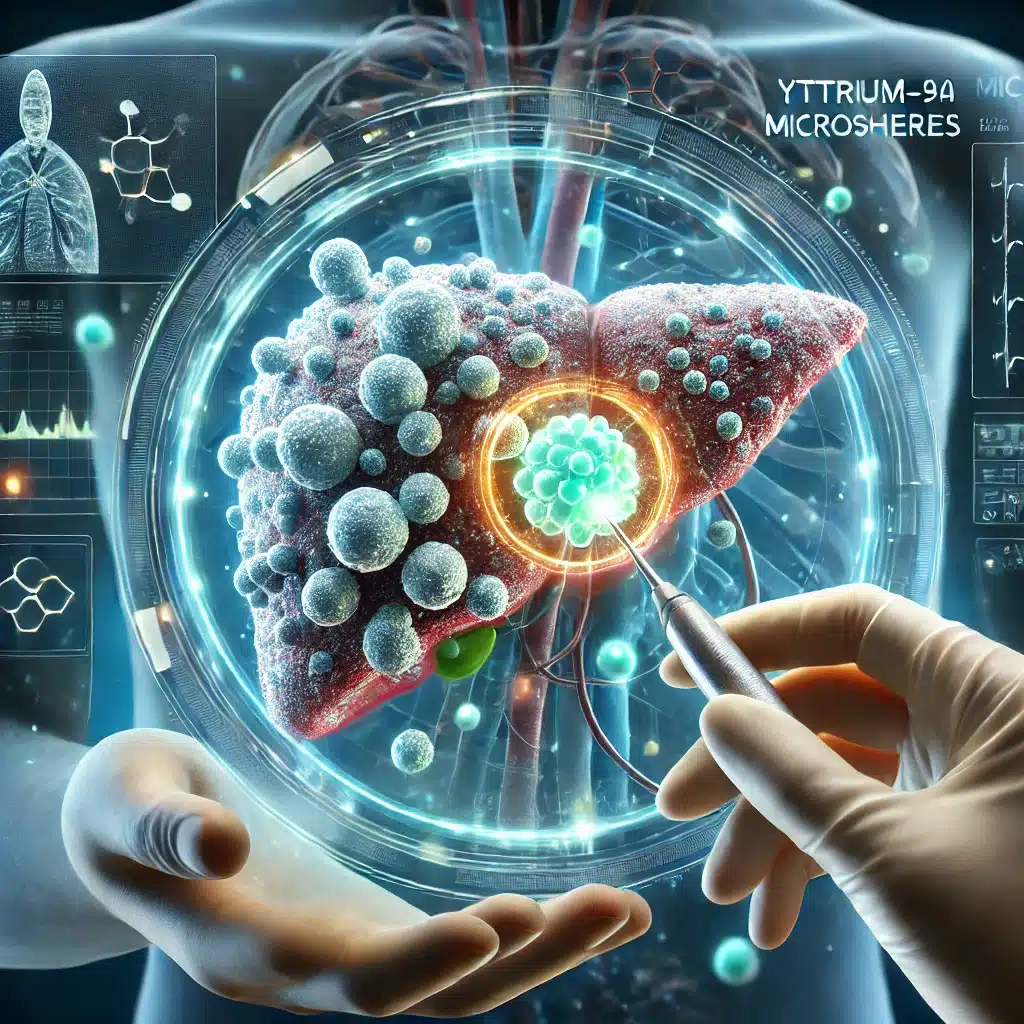
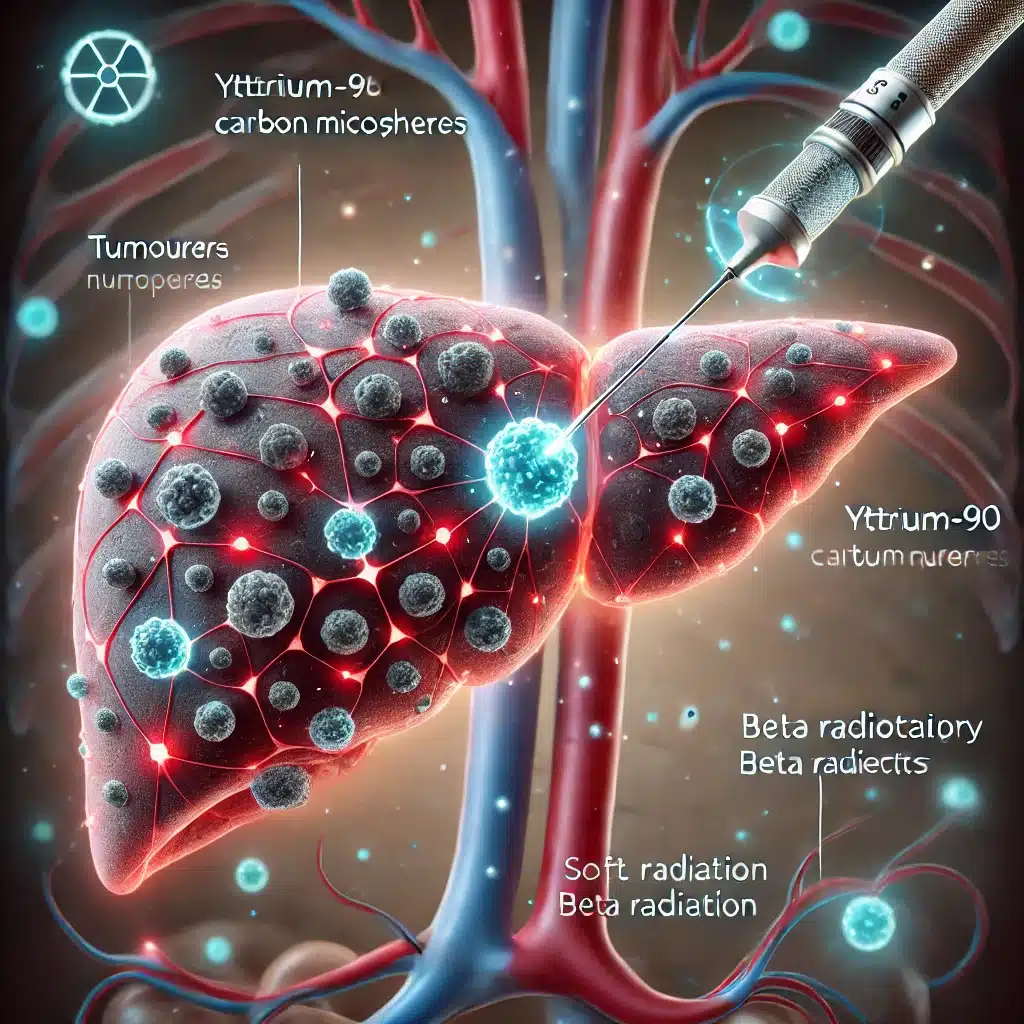
Beyond alpha emitters, beta-emitting radionuclides like Yttrium-90 (Y-90) and Lutetium-177 (Lu-177) are also making strides in radiotherapeutics. Y-90 is employed in treating hepatocellular carcinoma and non-Hodgkin’s lymphoma, while Lu-177 is used in therapies for prostate cancer and neuroendocrine tumours.
Expanding the repertoire, treatments like At-211-81C6 mAb (Neuradiab) target brain cancer by using Astatine-211, another alpha emitter, conjugated with the monoclonal antibody 81C6. Similarly, At-211-MABG, targeting adrenergic tissues, is being developed for conditions like paragangliomas and pheochromocytoma.
Furthermore, Radium-223 (Ra-223) in Radium Dichloride therapy stands out for its application in bone pain palliation, particularly for metastatic prostate cancer. This therapy mimics calcium and selectively targets bone metastases, delivering alpha radiation.
The field also sees the development of therapies like Iodine-131 (I-131) in various forms, such as I-131-Apamistamab (Iomab-B™) for acute lymphoblastic leukaemia and Hodgkin’s lymphoma and I-131-Metuximab for hepatocarcinoma. Each of these I-131-based therapies leverages the beta radiation emitted by I-131 to target specific cancer cells.
In conclusion, the diversity of radiotherapeutics, from alpha and beta emitters to different targeting mechanisms, underscores the remarkable progress in this field. These therapies are advancing and redefining cancer treatment, offering more precise, effective, and patient-tailored options. As research and clinical trials continue, these groundbreaking developments in radiotherapeutics are poised to significantly impact the future of cancer therapy.
You are here:
home » medical radiotherapeutics