Medical Imaging News
Medical Imaging News delivers timely updates on MRI, CT, PET, ultrasound, and AI, highlighting clinical research, technological innovations, regulatory changes, and breakthroughs transforming healthcare diagnostics, treatment strategies, and patient outcomes across global medicine.
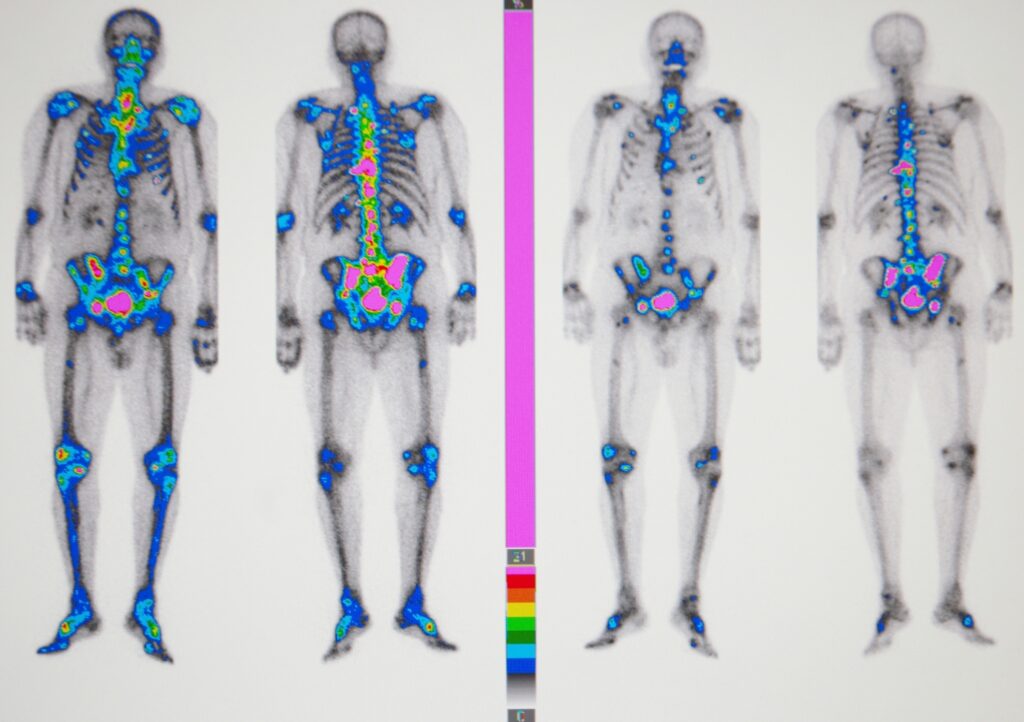
Radiology
Medical radiology employs diagnostic medical imaging technologies such as X-ray, ultrasound, and CT to support diagnosis, guide treatment, and perform minimally invasive procedures, enhancing accuracy, patient care, and clinical outcomes. Image for illustration purposes only. The person shown is a model.
Medical Science Liaison
Medical Science Liaison professionals build strong relationships with healthcare experts, communicate clinical data, and support safe, effective medication use, advancing medical knowledge and improving patient care outcomes. Image for illustration only. People depicted are models.
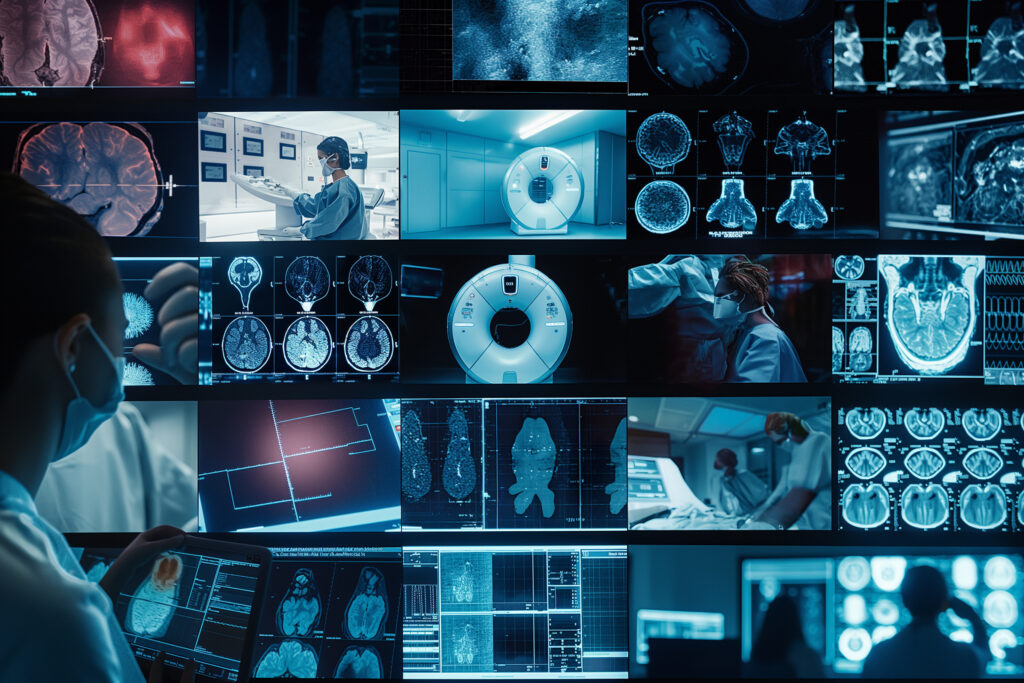
Medical Technologies
Advancements in diagnostic medical imaging technologies are transforming treatment and patient care, with innovations such as AI, next-generation imaging, and robotics delivering greater precision, efficiency, and improved outcomes across modern healthcare. Image for illustration purposes only. The person shown is a model.