Summary: Carbon-14 (14C) is a vital isotope in the field of radiochemistry due to its unique properties that make it particularly suitable for labelling complex molecules. This article outlines the key reasons for choosing carbon-14 for radiolabelling, exploring its chemical and physical advantages, applications in scientific research, and how it compares to other isotopes used in similar processes. The insights provided here will highlight the importance of 14C in various industries, including pharmaceuticals, environmental science, and biochemical studies.
Introduction to Radiolabelling
Radiolabelling is an essential tool in modern science, allowing researchers to trace the behaviour of specific molecules in complex systems. Among the various isotopes used, carbon-14 stands out as a preferred choice for many applications. Its advantageous properties and compatibility with biological and chemical systems make it indispensable for studying the intricate workings of molecules in research settings. This article will explore the fundamental reasons for choosing carbon-14 for radiolabelling, detailing its properties, benefits, and wide-ranging applications.
What Is Carbon-14?
Carbon-14 (14C) is a radioactive isotope of carbon with an atomic mass of 14. It contains six protons and eight neutrons, distinguishing it from the more stable and abundant carbon-12 isotope. 14C is naturally produced in the atmosphere through interactions between cosmic rays and nitrogen and is incorporated into living organisms through the carbon cycle. This incorporation allows scientists to trace carbon-14 in natural and experimental settings, making it highly valuable in radiolabelling applications.
Key Reasons for Choosing Carbon-14 for Radiolabelling
Long Half-Life
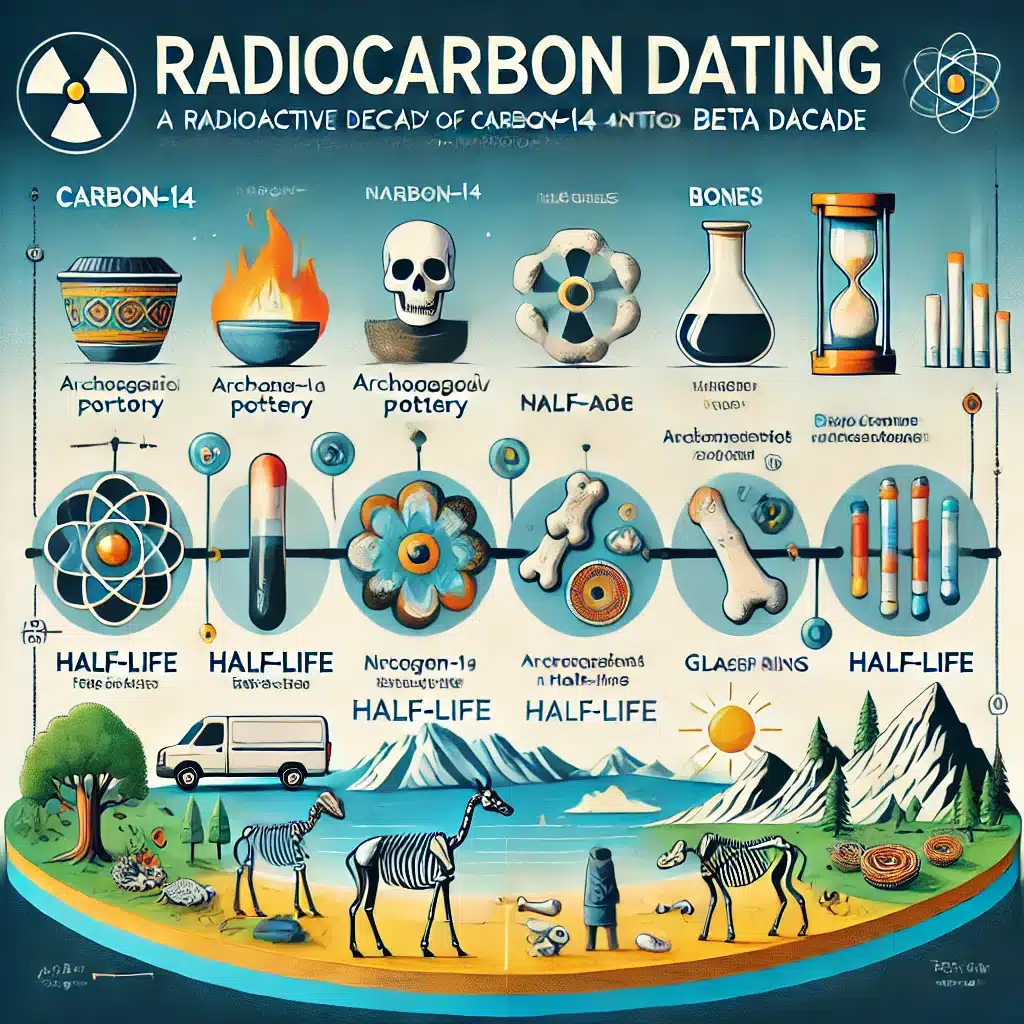
One of the primary reasons for selecting carbon-14 for radiolabelling is its relatively long half-life of approximately 5,730 years. This extended half-life ensures that labelled compounds remain stable and detectable over prolonged periods, which is particularly useful for long-term studies.
- Significance in Research: The long half-life of 14C makes it suitable for tracking biochemical and environmental processes that occur over weeks, months, or even years. This contrasts with isotopes like carbon-11 (11C), which has a half-life of just 20.4 minutes, limiting its application to shorter studies.
- Consistency in Results: The stability provided by 14C reduces the risk of significant decay during the experimental timeframe, ensuring consistent and reliable data collection.
Minimal Structural Impact
Carbon-14 is chemically identical to carbon-12 and carbon-13, which means incorporating 14C into a molecule does not significantly alter the compound’s chemical or physical properties.
- Preservation of Biological Properties: Since carbon-14 behaves similarly to the other carbon isotopes, labelled molecules retain their natural characteristics. This is crucial for studies involving biological pathways, where altering the structure could lead to misleading results.
- Natural Integration: The seamless integration of 14C into organic molecules makes it an excellent choice for studying complex biochemical reactions, as it ensures that the labelled compound acts like its unlabelled counterpart.
Versatility Across Various Applications
Carbon-14’s properties allow it to be used in a broad range of applications across multiple fields. From drug development to environmental tracing, its versatility is unmatched.
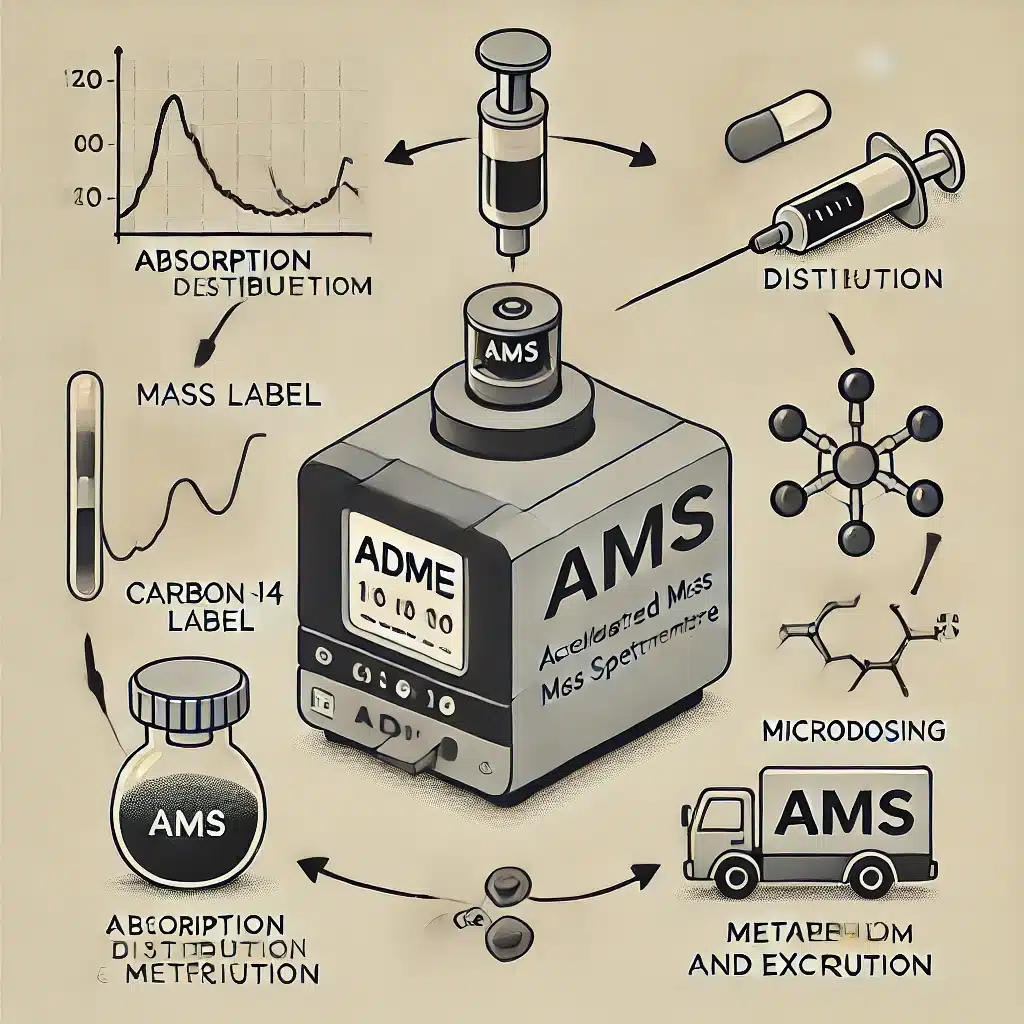
- Pharmaceutical Industry: 14C is widely used in drug development to study absorption, distribution, metabolism, and excretion (ADME) profiles. Radiolabelling with 14C helps researchers track the pathway of a drug within the body, identifying potential metabolites and understanding its efficacy.
- Environmental Studies: Carbon-14 is used to trace carbon flow in ecosystems, studying processes like photosynthesis and carbon sequestration. This helps scientists understand the impact of climate change and assess the carbon cycle.
- Archaeological Dating: Although not a direct application of radiolabelling, carbon-14 dating (radiocarbon dating) is a well-known use of the isotope, highlighting its importance in determining the age of organic materials.
Comparison with Other Isotopes
Radiolabelling can be achieved using various isotopes, each with its advantages and limitations. Understanding how carbon-14 compares with other isotopes provides insight into why it is a preferred choice for many researchers.
Carbon-11 (11C)
- Short Half-Life: With a half-life of only 20.4 minutes, 11C is primarily used in positron emission tomography (PET) imaging. Its rapid decay makes it suitable for short-term studies but limits its use in experiments requiring prolonged observation.
- Synthesis Challenges: The short half-life of 11C necessitates on-site production and immediate use, adding logistical complexity and cost to research.
Hydrogen-3 (Tritium, 3H)
- Shorter Half-Life: Tritium has a half-life of approximately 12.3 years, shorter than that of 14C but still suitable for medium-term studies.
- Beta Emission: Tritium emits low-energy beta particles, making it safe for many laboratory settings. However, its use can alter the physical properties of a compound more than 14C, which could affect experimental outcomes.
- Applications: Tritium is often used in labelling due to its simpler incorporation into molecules, but it is less ideal for studying carbon-specific interactions within biological systems.
Phosphorus-32 (32P) and Sulphur-35 (35S)
- Highly Specific Applications: These isotopes are more commonly used for labelling nucleic acids and proteins. They have shorter half-lives and higher energy emissions, which limit their use to specific types of studies and require stringent safety measures.
- Limited Versatility: Unlike carbon-14, 32P and 35S cannot be used as broadly across different types of organic molecules.
Safety and Handling of Carbon-14
Although 14C is a radioactive isotope, its beta emissions are of low energy, making it safer to handle compared to higher-energy isotopes. However, appropriate safety protocols must be followed to ensure minimal exposure and contamination.
- Containment: Working with 14C requires controlled environments with adequate ventilation to prevent the spread of contamination.
- Detection and Monitoring: The use of Geiger counters and liquid scintillation counting helps monitor and measure 14C levels during experiments, maintaining safety standards in the laboratory.
- Waste Management: Proper disposal of 14C-labelled waste is essential, adhering to regulations set by environmental and safety authorities to prevent contamination.
Methods of Incorporating Carbon-14 in Radiolabelling
There are several techniques for incorporating carbon-14 into molecules, which include:
Isotope Exchange
Isotope exchange involves replacing a stable carbon atom within a molecule with 14C without changing the overall structure of the compound. This method is particularly effective for labelling specific positions within complex molecules.
- Benefits: The original structure is retained, ensuring the labelled compound behaves similarly to its non-labelled version.
- Applications: Ideal for metabolic studies and research where positional labelling is critical for tracking specific carbon atoms.
Late-Stage Labelling
Late-stage labelling introduces 14C into a molecule after most of the synthetic process is complete. This technique allows for labelling at specific positions with high selectivity and minimal impact on the molecule’s synthesis route.
- Advantages: This method reduces the time and cost involved in synthesising a compound from scratch.
- Efficiency: Late-stage labelling is well-suited for the development of radiolabelled drugs where modifications to existing compounds are necessary.
Carbon-14 Precursor Synthesis
Labelling using 14C precursors involves the incorporation of carbon-14 during the initial stages of molecule synthesis. This approach is beneficial for creating labelled compounds from the ground up, providing more control over the positioning of the isotope.
- Drawbacks: While highly effective for specific needs, this method can be time-consuming and resource-intensive compared to late-stage labelling.
Applications of Carbon-14 Radiolabelling in Modern Research
Pharmaceutical Development
The pharmaceutical industry relies heavily on 14C labelling to understand the pharmacokinetics and pharmacodynamics of new drugs. Radiolabelled compounds help researchers determine how drugs are absorbed, distributed, metabolised, and excreted in the body, ensuring safe and effective treatments.
Clinical Trials: 14C-labelled drugs are used in microdosing studies, where minute amounts of the drug are administered to human subjects to gather early pharmacokinetic data without significant risk.
Environmental and Climate Research
Radiolabelling with 14C plays an essential role in tracking the movement of carbon through natural systems. By studying the incorporation and movement of 14C in plants, soils, and water bodies, researchers can gain insights into the carbon cycle and better understand environmental changes and carbon sequestration efforts.
Biochemical Pathways and Metabolic Studies
In biochemistry, 14C labelling allows scientists to map metabolic pathways and identify intermediate compounds in complex reactions. By incorporating 14C-labelled substrates, researchers can trace the transfer of carbon atoms through various biochemical processes and better understand cellular functions.
Conclusion
The choice of carbon-14 for radiolabelling is rooted in its exceptional properties, including a long half-life, minimal structural impact on labelled compounds, and versatility in applications across different research fields. Compared to other isotopes, 14C provides a balance of stability, safety, and utility, making it an invaluable tool for scientific discovery. From drug development and metabolic studies to environmental research, the use of carbon-14 continues to pave the way for significant advancements in our understanding of complex molecular systems. With ongoing innovation in radiolabelling techniques, the future promises even greater precision and efficiency in how we study the dynamic world of chemistry and biology.
Disclaimer
The content provided in this article is intended for informational and educational purposes only. While every effort has been made to ensure the accuracy and reliability of the information presented, Open Medscience makes no representations or warranties of any kind, express or implied, regarding the completeness, accuracy, or suitability of the information for any specific purpose.
This article does not constitute professional advice in radiochemistry, pharmaceuticals, environmental science, or any other related discipline. Readers are advised to consult qualified professionals or regulatory bodies before applying any of the concepts or techniques discussed, especially in laboratory or clinical settings.
Open Medscience and the authors disclaim any liability for any loss or damage arising directly or indirectly from the use or reliance on the information provided in this publication.
You are here: home » diagnostic medical imaging blog »