Radionuclides
Radionuclides, or radioactive isotopes, are atoms with an unstable nucleus. They can undergo radioactive decay, emitting ionizing radiation in the form of alpha, beta, or gamma particles. Radioactive isotopes have many applications in diverse fields, such as medicine, industry, agriculture, and environmental research. Throughout this article, we will explore radioisotopes’ properties, production, and applications, along with the potential risks and benefits associated with their use.
The unstable nucleus of a radionuclide contains a unique combination of protons and neutrons, which leads to an imbalance in the nuclear forces. This instability causes the nucleus to undergo radioactivity, resulting in the emission of ionising radiation. The time it takes for half of a radionuclide sample to decay is known as its half-life, ranging from a fraction of a second to millions of years. Some common radionuclides include carbon-14, uranium-238, and iodine-131.
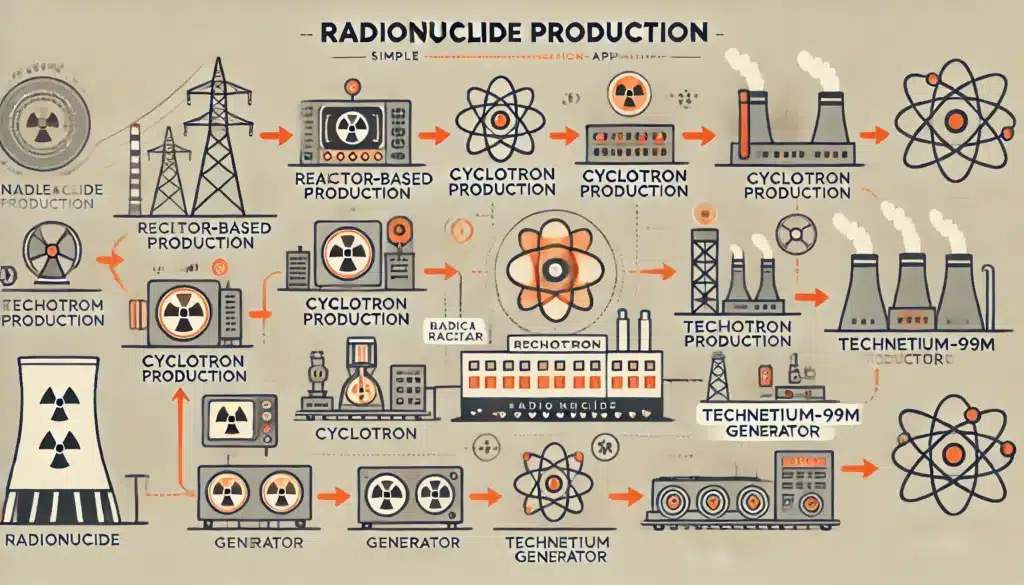
Radionuclides can be produced through several methods, including:
- Radionuclides can be generated by bombarding stable isotopes with charged particles, such as protons or neutrons. This process occurs in particle accelerators, reactors, or cyclotrons, transforming the stable isotope into a radioactive one.
- In nuclear fission, heavy nuclei, such as uranium-235 or plutonium-239, split into smaller nuclei, releasing energy and creating a variety of radionuclides as fission products.
- Some radioisotopes are naturally occurring, originating from the decay of long-lived isotopes in the Earth’s crust, such as uranium and thorium.
Radionuclides have a wide range of applications across various fields:
- In nuclear medicine, radioisotopes are used for diagnostic imaging (e.g., PET scans) and targeted therapy (e.g., radioimmunotherapy). Radioactive tracers can help visualise organ function, detect tumours, and deliver therapeutic doses of radiation directly to cancer cells.
- Industrial applications of radioisotopes include radiography, which detects defects in materials, measures thickness or density, and tracks the movement of fluids in pipelines.
- Radionuclides can be used to study nutrient uptake and transport in plants, improving crop production and fertiliser efficiency.
- Radionuclides are valuable tools for tracking pollution sources, determining sedimentation rates, and dating geological samples.
Risks and Benefits of Radioisotopes
The use of radionuclides carries both risks and benefits. The ionising radiation emitted by radioisotopes can cause harm to living organisms, potentially damaging cells and DNA. However, radionuclides offer significant benefits in various applications when used responsibly and with appropriate safety measures.
You are here:
home » radionuclides