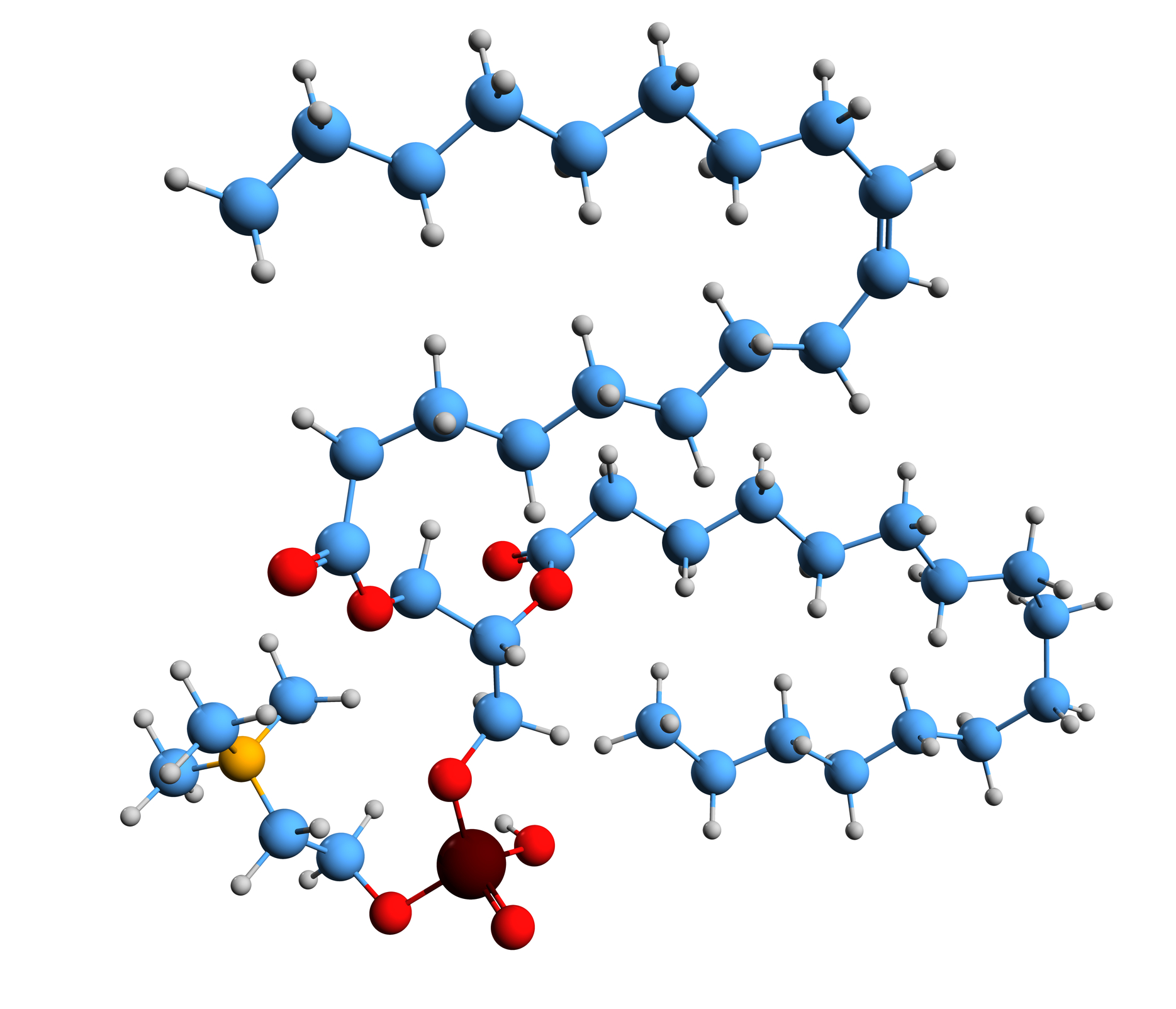
Peptides have emerged as pivotal molecular tools within diverse research fields due to their versatility in modulating cellular processes. Among these, Palmitoyl Tripeptide-1, a synthetic lipopeptide consisting of a palmitoyl chain conjugated to a specific tripeptide sequence, has garnered considerable interest.
This peptide is notable for its structural mimicry of certain extracellular matrix (ECM) components, supporting its interactions with cell surface receptors and modulate signalling pathways associated with tissue remodelling and regeneration. This article examines the properties of Palmitoyl Tripeptide-1, explores its proposed mechanisms of action, and discusses its potential implications in research, encompassing cellular biology, tissue engineering, and biomaterial development.
Introduction to Palmitoyl Tripeptide-1
Palmitoyl Tripeptide-1 is characterised by a fatty acid (palmitic acid) linked to a short peptide chain, typically composed of three amino acids that resemble fragments of procollagen or ECM proteins such as type I collagen. This structural design is believed to help the peptide showcase better-supported lipophilicity, thereby supporting its interaction with cellular membranes and potential receptor targets.
It has been theorised that the lipid modification may promote the peptide’s stability and facilitate its integration into cell membrane microdomains, where it might support signal transduction processes. Given its resemblance to ECM sequences, the peptide is believed to act as a biomimetic agent, triggering cellular responses that encourage ECM synthesis, remodelling, and repair.
Molecular and Cellular Properties
ECM Modulation and Collagen Synthesis
A central hypothesis in Palmitoyl Tripeptide-1 research purports that the peptide may function as a molecular signal mimicking damaged ECM fragments. This mimicry might induce receptor-mediated pathways that promote the production of structural proteins, particularly types I and III collagen, which are fundamental to the mechanical integrity and elasticity of connective tissues.
Research models suggest that exposure to this peptide may upregulate gene expression associated with collagen precursors and matrix metalloproteinases (MMPs), enzymes involved in the remodelling of the ECM. This dynamic remodelling might serve to maintain or restore tissue homeostasis, potentially through feedback mechanisms that sense and respond to microenvironmental cues.
Possible Support for Fibroblast Activity
Fibroblasts, the primary cellular architects of the ECM, might showcase altered behaviour in the presence of Palmitoyl Tripeptide-1. Studies suggest that the peptide may stimulate fibroblast proliferation, migration, and the synthesis of matrix components by activating intracellular pathways, such as the mitogen-activated protein kinase (MAPK) cascade or the transforming growth factor-beta (TGF-β) pathway. These pathways are well known for regulating cellular growth and matrix production.
Consequently, the peptide’s interaction with fibroblasts is thought to facilitate better-supported matrix deposition and remodelling processes, crucial for tissue regeneration and wound closure in laboratory settings.
Possible Implications in Research Domains
Tissue Engineering and Regenerative Science
In tissue engineering, scaffolds that promote cellular attachment and matrix deposition are essential for developing functional tissue constructs. Research indicates that Palmitoyl Tripeptide-1 may be integrated into biomaterial scaffolds as a bioactive cue, potentially stimulating ECM synthesis and promoting cellular organisation within three-dimensional matrices.
Research suggests that the peptide’s lipidation may facilitate its incorporation into hydrogels or nanofiber scaffolds, thereby supporting their bioactivity without compromising structural integrity. By mimicking ECM components, the peptide seems to support cell-scaffold interactions, thereby fostering a regenerative microenvironment conducive to tissue repair or growth.
Murine Wound Research
The dynamic modulation of collagen and fibroblast activity by Palmitoyl Tripeptide-1 suggests its relevance in models studying tissue repair and wound healing in murine models. Investigations purport that the peptide might accelerate matrix remodelling, promote the deposition of structural proteins, and support cellular migration into wound sites.
In controlled research models, the peptide’s potential to support the balance between collagen synthesis and degradation might provide insight into optimal conditions for scarless dermal healing and regeneration of dermal cells. Its support for signalling pathways implicated in inflammation and repair might also be a subject of investigative interest.
Anti-Cellular Ageing and Dermatological Research
Although this area traditionally focuses on relevant research, basic research using Palmitoyl Tripeptide-1 may explore its potential support for ECM homeostasis in dermal cell models. The peptide’s potential to stimulate collagen production may inform studies on dermal cell ageing, elasticity, and repair processes. This may include investigations into molecular mechanisms underlying tissue resilience and responses to environmental stressors such as ultraviolet radiation.
Furthermore, the peptide has been hypothesised to serve as a model molecule for understanding how ECM signalling supports cellular senescence and proliferation, shedding light on cellular age-associated changes in tissue architecture.
Mechanistic Insights and Hypothesised Pathways
The specific receptor interactions of Palmitoyl Tripeptide-1 remain an active area of research. It has been hypothesised that the peptide may bind to integrins or other ECM receptors on the cell surface, triggering downstream cascades that regulate gene transcription relevant to matrix synthesis.
The palmitoyl moiety might assist in targeting lipid raft microdomains, facilitating receptor clustering and supporting signal specificity and intensity. This may support various intracellular pathways, including:
- Activation of focal adhesion kinase (FAK) promotes cytoskeletal reorganisation and cellular migration.
- Modulation of TGF-β signalling, a critical regulator of ECM synthesis and fibroblast activation.
- Induction of nuclear transcription factors such as AP-1 and NF-κB, which might govern gene expression related to cell proliferation and matrix protein production.
Potential for Combinatorial Implications and Synergistic Research
Given the complexity of ECM dynamics, Palmitoyl Tripeptide-1 has been theorised to be employed in conjunction with other peptides or growth factors to replicate the multifaceted environment of tissue regeneration. For example, co-exposure with peptides that stimulate angiogenesis or modulate inflammatory responses might yield synergistic supports for repair processes.
Findings imply that the peptide’s properties may also facilitate its inclusion in multi-component biomaterials designed for staged release of bioactive molecules, thereby optimising temporal and spatial signalling within engineered tissues.
Conclusion
Palmitoyl Tripeptide-1 emerges as a promising peptide in research domains focusing on ECM modulation, tissue regeneration, and biomaterial functionalization. Its molecular design, which combines lipidation with a bioactive peptide sequence, has been speculated to support unique interactions with cellular receptors and signalling pathways that govern matrix synthesis and remodelling.
The peptide’s properties suggest it might serve as a powerful agent for stimulating fibroblast activity, supporting ECM deposition, and facilitating tissue repair in research models. Its potential for incorporation into biomaterials offers further avenues for exploration in regenerative and reconstructive sciences.
While the mechanistic details continue to unfold, Palmitoyl Tripeptide-1 represents a compelling candidate for diverse investigative contexts, paving the way for novel biomimetic approaches in understanding and manipulating tissue architecture at the molecular level. For more useful peptide data, visit this article.
Disclaimer
The information provided in this article, “Palmitoyl Tripeptide-1: Exploring Its Multifaceted Potential in Research Domains”, is intended solely for educational and research-related purposes. It is not intended to serve as medical advice, diagnosis, or treatment guidance. Palmitoyl Tripeptide-1 and related compounds discussed herein are to be considered as subjects of ongoing scientific investigation, and their effects, safety, and efficacy have not been fully established in clinical or therapeutic settings.
Readers are advised that any reference to biological activity, laboratory outcomes, or theoretical mechanisms is based on preclinical or experimental research data and should not be interpreted as evidence of clinical benefit or safety for human use. Open MedScience does not endorse, promote, or encourage the use of Palmitoyl Tripeptide-1 or any peptide-based compound for medical, cosmetic, or commercial purposes outside of regulated and ethical research environments.
Researchers and readers should ensure compliance with all applicable laws, institutional guidelines, and ethical standards when handling, studying, or referencing materials of this nature. Open MedScience assumes no responsibility for any interpretation, application, or consequence resulting from the information presented in this article.
home » blog » medical technologies »