Summary: Radiocarbon, specifically carbon-14, has become an invaluable tool across various fields of chemistry due to its unique properties and decay characteristics. This article explores the fundamental principles of radiocarbon, its applications in analytical and synthetic chemistry, and its impact on areas such as archaeology, environmental science, and pharmaceutical development. By reviewing the techniques used for radiocarbon measurement and tracing, we can appreciate the diverse ways radiocarbon has transformed modern chemical research and practical applications.
Introduction to Radiocarbon Chemistry
Radiocarbon, or carbon-14 (C-14), is a radioactive isotope of carbon that has transformed our understanding of organic materials and dating processes. Discovered in the 1940s, radiocarbon dating was initially used to date ancient artefacts and geological samples. However, its utility extends beyond archaeology into various branches of chemistry, from environmental studies to pharmacokinetics. Carbon-14 has a half-life of about 5,730 years, making it suitable for studying both ancient and more recent samples, allowing chemists to trace the path of carbon atoms in diverse systems.
Radiocarbon Production and Natural Occurrence
Carbon-14 is naturally formed in the atmosphere through interactions between nitrogen atoms and cosmic rays. These cosmic rays convert nitrogen-14 (N-14) atoms into carbon-14, which is then incorporated into carbon dioxide and distributed throughout the biosphere. Living organisms assimilate this carbon-14 through respiration or photosynthesis, creating a dynamic equilibrium in which the concentration of C-14 remains relatively constant in all living things.
Upon the death of an organism, the uptake of C-14 ceases, and the existing C-14 atoms begin to decay. By measuring the remaining C-14 in organic material, scientists can estimate the age of the sample, a technique known as radiocarbon dating. This foundational principle has paved the way for numerous applications within chemistry and other scientific fields.
Applications of Radiocarbon in Chemistry
Radiocarbon Dating in Archaeology and Geochemistry
One of the most well-known applications of radiocarbon is radiocarbon dating, which revolutionised archaeology by providing a reliable method for dating organic materials. In geochemistry, radiocarbon dating aids in understanding sedimentary processes and tracing carbon sources and reservoirs. Chemical analysis of radiocarbon content in geological samples, for instance, helps map carbon cycles and understand atmospheric changes over millennia.
Radiocarbon dating also allows chemists to study the age of geological formations containing organic material, offering insights into past environmental conditions and aiding in climate change research.
Environmental Chemistry and Carbon Cycle Studies
Environmental chemists use radiocarbon to track carbon flows within ecosystems. By measuring radiocarbon levels in soil, water, and atmospheric samples, researchers can assess carbon sources, determine fossil fuel contributions, and study biological carbon cycling. Radiocarbon data, combined with stable isotopes, enables a clearer understanding of how carbon moves through different environmental reservoirs and how human activities impact these cycles.
One critical area of study is the “bomb carbon” effect, where atmospheric nuclear tests from the mid-20th century doubled atmospheric C-14 levels. This excess radiocarbon entered the carbon cycle and serves as a useful tracer in environmental studies. Radiocarbon signatures from this period help track how carbon is absorbed by oceans, taken up by plants, and eventually cycled back into the atmosphere.
Radiocarbon in Pharmaceutical Chemistry
Radiocarbon-labelled compounds are invaluable in pharmaceutical development, enabling pharmacokinetics and drug metabolism studies. By incorporating C-14 into a drug molecule, scientists can trace its metabolic pathways, allowing them to identify metabolites, monitor bioavailability, and evaluate drug efficacy and safety. Radiolabelled compounds with C-14 provide essential insights that inform dosing, toxicity, and bioaccumulation potential in new drugs.
C-14 tracing also assists in understanding how drugs interact with different tissues and organs. This capability is particularly useful for drugs that have long-lasting effects or slow metabolism, as C-14’s longer half-life allows for extended studies compared to other radiolabels.
Synthetic Chemistry and Mechanistic Studies
In synthetic chemistry, radiocarbon labelling aids in understanding reaction mechanisms by tracking specific atoms within a molecule. By using C-14-labelled precursors, chemists can map out reaction pathways, monitor intermediate compounds, and clarify ambiguous steps in complex reactions. These insights are crucial in developing new chemical reactions and improving efficiency in synthetic processes.
Radiocarbon tracing also supports isotope dilution methods, where chemists use known quantities of a radiolabelled compound to quantify unknown amounts in a sample. This application is particularly valuable in organic synthesis and industrial chemistry.
Techniques for Measuring Radiocarbon
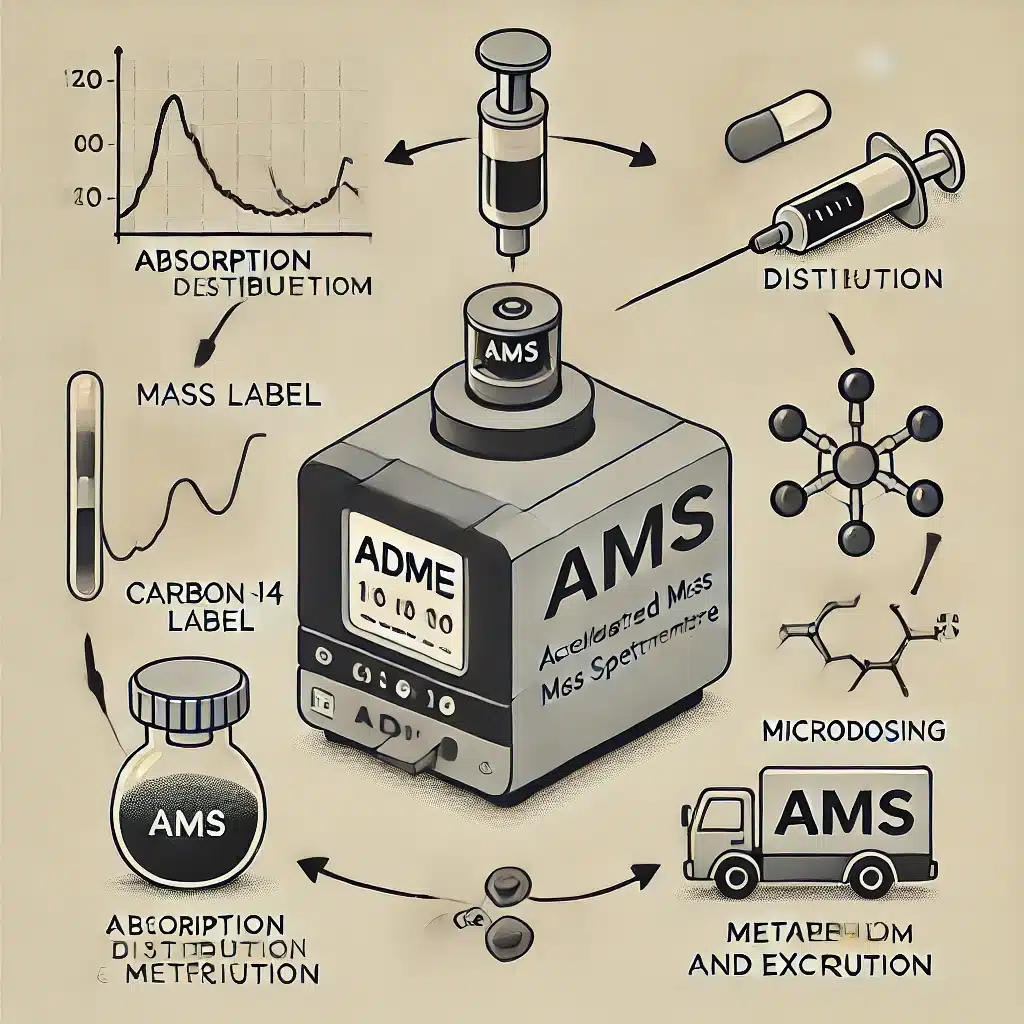
Accelerator Mass Spectrometry (AMS)
Accelerator Mass Spectrometry (AMS) is the most precise method for measuring C-14. AMS accelerates ions to extremely high kinetic energies, separating C-14 from other carbon isotopes and allowing for detection of individual atoms. This sensitivity enables measurements from minute samples, such as individual seeds, hair strands, or tiny fragments of biological tissue. AMS revolutionised radiocarbon dating by reducing the amount of material required, thus preserving precious archaeological and biological samples.
In chemistry, AMS allows researchers to trace radiocarbon-labelled compounds at extraordinarily low concentrations. Its high precision is particularly advantageous in environmental and pharmaceutical studies, where accurate tracing of C-14 is essential.
Liquid Scintillation Counting (LSC)
Liquid scintillation counting is a widely used technique for measuring radiocarbon in solution. The sample is dissolved in a scintillation cocktail, where the C-14 beta particles excite the solvent, emitting photons that are detected by photomultiplier tubes. LSC is suitable for samples with relatively higher C-14 concentrations, although it is less sensitive than AMS and requires larger samples.
LSC remains useful in fields such as drug development, where C-14-labelled compounds can be detected in bodily fluids or organic extracts. It also plays a role in environmental studies, where larger samples such as soil extracts are commonly available.
Gas Proportional Counting (GPC)
Gas proportional counting measures C-14 by detecting beta radiation in gaseous CO2 samples. This technique involves converting the sample’s carbon into CO2 gas, which is then measured in a proportional counter filled with argon or methane. While GPC is less sensitive than AMS and not suitable for trace analysis, it is still used for radiocarbon dating of samples with moderate C-14 concentrations, such as organic sediments or groundwater samples.
In chemistry, GPC finds application in scenarios where sample material is readily available and where precise measurements are not as critical, such as in preliminary studies.
Radiocarbon and Stable Isotope Analysis: Complementary Approaches
Radiocarbon analysis is often used in conjunction with stable isotope analysis, which measures non-radioactive isotopes like carbon-12 and carbon-13. Stable isotope ratios provide information on the sources and processes affecting carbon compounds, while radiocarbon adds a temporal dimension, revealing the age or turnover of carbon in a sample. Together, these approaches offer a comprehensive understanding of carbon cycling in environmental chemistry, allowing scientists to differentiate between recent and fossil carbon sources and assess ecosystem responses to climate change.
For example, radiocarbon and stable isotopes together reveal how long carbon remains stored in different pools, such as soil organic matter, ocean sediments, or plant biomass. These insights contribute to modelling global carbon cycles and understanding the longevity of carbon sequestration efforts.
Challenges and Future Directions in Radiocarbon Chemistry
Sample Preparation and Contamination
Accurate radiocarbon measurement depends on meticulous sample preparation, as contamination with modern carbon can skew results. Laboratories must take steps to eliminate or account for potential contaminants, particularly when working with minute or ancient samples. In pharmaceutical chemistry, careful labelling and isolation procedures are essential to prevent cross-contamination in drug testing.
Advancements in Analytical Methods
Future advancements in radiocarbon measurement may further reduce sample requirements and increase sensitivity. Newer techniques under development, such as cavity ring-down spectroscopy (CRDS) and laser-based approaches, offer non-destructive alternatives to traditional methods. These innovations could broaden radiocarbon’s application to new fields, such as micro-analyses of individual cells or nanomaterials.
Expanding Applications in Medical and Forensic Sciences
Radiocarbon’s potential is expanding in medical diagnostics and forensic sciences. In medical research, C-14 labelling can assist in studying long-term drug accumulation and metabolic rates, particularly in age-related diseases or chronic conditions. In forensics, radiocarbon dating can be used to estimate the time of death in forensic cases, providing new insights into tissue decay and turnover.
Ethical and Environmental Considerations
The use of radiocarbon in chemistry is not without ethical and environmental concerns. The production of radiolabelled compounds requires handling radioactive material, which must be managed responsibly to protect researchers and minimise environmental impact. Strict regulations and safety protocols govern the use, disposal, and storage of C-14-labelled materials in laboratory settings.
Additionally, radiocarbon research that involves human or animal testing must adhere to ethical guidelines that ensure the safety and well-being of participants. As radiocarbon applications expand, particularly in biomedical fields, ongoing discussions on ethical use and long-term environmental impacts will remain crucial.
Conclusion
Radiocarbon has proven to be a versatile and invaluable tool across diverse fields of chemistry. From dating ancient artefacts to tracing carbon pathways in environmental systems, radiocarbon offers unique insights that are unattainable with other techniques. Advances in measurement techniques like AMS have expanded radiocarbon applications, allowing researchers to work with smaller samples and higher precision, thus broadening its impact across scientific disciplines.
In pharmaceutical and synthetic chemistry, radiocarbon labelling has become a foundational technique for tracing molecular interactions, understanding metabolic pathways, and developing safer and more effective drugs. The combination of radiocarbon dating with stable isotope analysis has enabled chemists and environmental scientists to gain a holistic view of carbon dynamics, enhancing our understanding of global carbon cycles and the long-term impacts of human activities on ecosystems.
Future directions in radiocarbon research promise further integration into areas such as nanotechnology, cellular biology, and forensic sciences. Techniques under development, like laser-based isotope analysis, could offer unprecedented precision, making it possible to conduct micro-level analyses that were previously impossible. Such advancements could pave the way for innovative applications in fields like single-cell analysis, nanomaterial studies, and personalised medicine, where tracing individual molecules within cells could revolutionise diagnostics and treatment strategies.
However, as with any powerful scientific tool, radiocarbon usage must be balanced with careful consideration of ethical implications and environmental safety. The handling and disposal of radiolabelled materials require stringent controls to prevent radiation exposure and contamination, ensuring that radiocarbon’s benefits are not offset by adverse environmental impacts. As researchers push the boundaries of radiocarbon applications, maintaining ethical standards and prioritising safety will be essential to sustaining its value as a tool in scientific exploration.
Radiocarbon has fundamentally reshaped our ability to trace, date, and understand carbon-based processes. Its applications in chemistry are vast, with uses that span environmental science, archaeology, synthetic chemistry, and pharmaceuticals. The continued development of radiocarbon techniques promises even greater insights, enabling chemists and scientists to uncover the molecular mysteries of past, present, and future carbon cycles. Radiocarbon’s role in chemistry exemplifies the power of isotope analysis in enhancing our understanding of natural and synthetic processes, offering a bridge between history, present-day challenges, and future scientific innovation.
Disclaimer
The information provided in Radiocarbon Revolution: Tracing Chemistry Across Time and Applications is intended for educational and informational purposes only. While every effort has been made to ensure the accuracy of the content at the time of publication, Open Medscience makes no representations or warranties regarding the completeness, reliability, or suitability of the information for any specific purpose.
This article does not constitute professional scientific advice or guidance. Readers are advised to consult appropriate specialists or regulatory authorities when handling radiolabelled materials or conducting research involving radioactive isotopes. Any experimental procedures or techniques involving carbon-14 should be carried out in accordance with relevant health, safety, and ethical regulations.
Open Medscience disclaims any liability for loss, injury, or damage arising from the use of the information contained in this publication. The views and interpretations expressed are those of the authors and do not necessarily reflect those of any affiliated institutions or regulatory bodies.
Use of radiocarbon and associated methodologies should always be undertaken responsibly, with due consideration for environmental and ethical implications.
home » blog » radiolabelling »