Summary: Carbon-14 (C-14) is a radioactive isotope of carbon that plays a pivotal role in various scientific fields, particularly in archaeology and environmental science. This article explores the formation, properties, applications, and significance of carbon-14 and how it is used for radiocarbon dating, the natural processes that sustain it, and the implications of its presence in the environment.
Introduction to Carbon-14
Carbon-14, symbolised as 14C, is a naturally occurring radioactive isotope of carbon. Unlike the more common carbon-12, carbon-14 contains two additional neutrons, giving it unique properties. It is unstable, decaying over time to nitrogen-14 through beta decay. This characteristic makes carbon-14 essential for scientific applications, especially for dating ancient artefacts and understanding environmental processes.
Formation of Carbon-14
Radioactive carbon-14 is formed in the upper atmosphere through the interaction of cosmic rays with nitrogen atoms. High-energy neutrons from cosmic radiation collide with nitrogen-14 (14N), causing it to lose a proton and transform into carbon-14:
14N + n → 14C + p
Once formed, carbon-14 becomes incorporated into carbon dioxide, which is then absorbed by living organisms during processes such as photosynthesis and respiration.
The Properties of Carbon-14
- Radioactive Nature: Carbon-14 is radioactive, with a half-life of approximately 5,730 years. This half-life is long enough to measure ages of up to 50,000 years with reasonable accuracy.
- Decay Process: Carbon-14 decays by emitting a beta particle, converting itself into nitrogen-14. The equation for this decay is:
14C → 14N + β− + νe
- Occurrence: Carbon-14 is found in trace amounts in the atmosphere and living organisms. Once an organism dies, it no longer absorbs carbon-14, causing the existing carbon-14 in its tissues to begin decaying at a known rate.
Applications of Carbon-14
Radiocarbon Dating
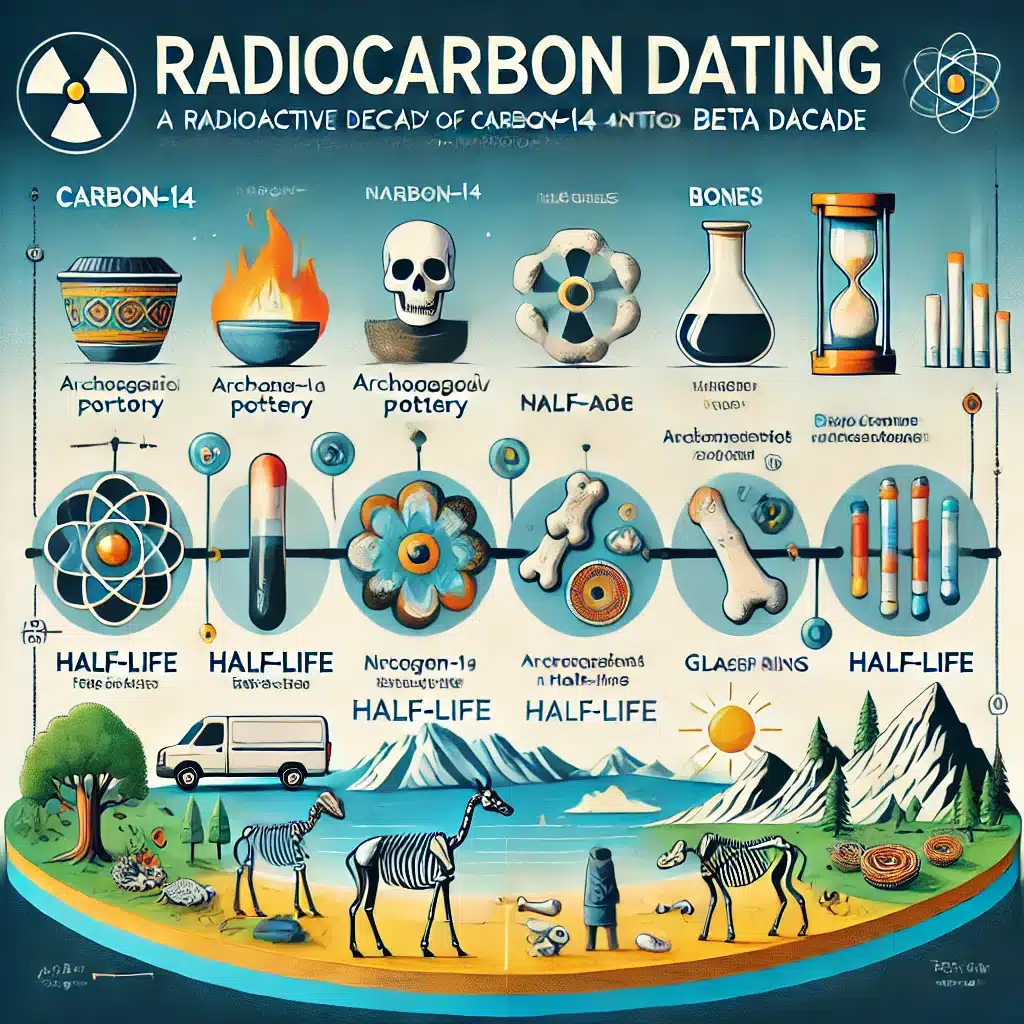
One of the most well-known applications of carbon-14 is radiocarbon dating, a technique used to determine the age of ancient biological materials. Developed by Willard Libby in the late 1940s, radiocarbon dating has revolutionised the field of archaeology by providing a method to date organic remains.
How It Works: When an organism is alive, it maintains a steady ratio of carbon-14 to carbon-12 by continuously exchanging carbon with its environment. When the organism dies, it stops absorbing carbon-14, and the isotope begins to decay. By measuring the remaining carbon-14 in a sample and comparing it to the known half-life, scientists can estimate the time of death.
Applications in Archaeology: Radiocarbon dating has been instrumental in dating artefacts such as wooden tools, bones, and textiles. This method has helped verify the timelines of historical events and understand the development of early civilisations.
Environmental Science and Climate Studies
Carbon-14 also plays an essential role in studying climate change and carbon cycles. By tracking the movement of carbon-14 in the environment, scientists can better understand how carbon is transferred between the atmosphere, oceans, and terrestrial ecosystems. This insight helps build accurate models of global carbon dynamics and assess the impact of human activities on the environment.
The Natural Carbon Cycle and Carbon-14 Decay
Carbon-14 is a key component of the global carbon cycle, which describes how carbon moves through the Earth’s biosphere, atmosphere, and oceans. The cycle can be summarised in three main processes:
- Absorption by Plants: Through photosynthesis, plants take in carbon dioxide containing carbon-14. Animals that consume these plants also take in carbon-14, incorporating it into their bodies.
- Respiration and Decay: Living organisms release carbon dioxide back into the atmosphere through respiration. When an organism dies, decomposition releases carbon back into the environment.
- Ocean Uptake: The world’s oceans absorb significant amounts of carbon dioxide, including carbon-14. This absorbed carbon can stay in the deep ocean for thousands of years, contributing to long-term carbon storage.
Factors Affecting Carbon-14 Levels
Various factors can influence the amount of carbon-14 in the atmosphere and living organisms:
- Cosmic Ray Activity: Changes in solar activity or geomagnetic field strength can alter the rate at which carbon-14 is produced in the atmosphere.
- Human Activities: The burning of fossil fuels, which contain no carbon-14, dilutes the concentration of carbon-14 in the atmosphere. This phenomenon, known as the Suess effect, can skew radiocarbon dating results.
- Nuclear Testing: Atmospheric nuclear tests in the mid-20th century significantly increased carbon-14 levels, creating a peak in radiocarbon known as the “bomb pulse.”
Limitations of Radiocarbon Dating
While radiocarbon dating is an invaluable tool, it has certain limitations:
- Age Range: Radiocarbon dating is most effective for samples up to 50,000 years old. Beyond this range, the remaining carbon-14 is too minimal to measure accurately.
- Contamination: Samples can be contaminated with modern carbon or other substances, leading to inaccurate results.
- Calibration: The amount of carbon-14 in the atmosphere has not remained constant over time. To address this, radiocarbon dates must be calibrated against tree rings and other data to obtain more accurate results.
The Significance of Carbon-14 in Modern Science
Carbon-14 has proven invaluable not only for dating historical artefacts but also for enhancing our understanding of carbon cycles, tracing the sources of pollution, and assessing the age of water sources in hydrology. Furthermore, carbon-14 is used in biomedicine to trace metabolic pathways and understand the mechanisms of various biological processes.
The Future of Carbon-14 Research
Advancements in technology continue to improve the precision of carbon-14 measurements. Accelerator Mass Spectrometry (AMS), for instance, has increased the sensitivity and accuracy of carbon-14 detection, allowing for the dating of smaller and more delicate samples. As research progresses, carbon-14 will likely play an even more prominent role in studying climate change, verifying the authenticity of art and historical objects, and monitoring biogeochemical cycles.
Conclusion
Carbon-14 is an extraordinary isotope that has transformed our understanding of history, ecology, and environmental science. Its formation in the atmosphere, integration into living organisms, and gradual decay form the basis for its primary application—radiocarbon dating. Through ongoing research and technological advancements, carbon-14 continues to shed light on the past, contribute to climate science, and provide insights into natural processes.
By understanding the properties and implications of carbon-14, scientists and researchers can continue to uncover the mysteries of the past and make informed predictions about the future of our planet.
Disclaimer
The content provided in this article, What is Radioactive Carbon-14? Its Formation, Uses, and Significance, is for informational and educational purposes only. Open Medscience does not offer any warranty or guarantee regarding the accuracy, completeness, or reliability of the information presented. While efforts have been made to ensure the scientific accuracy of the material, the article should not be considered a substitute for professional advice or consultation in scientific, archaeological, environmental, or medical fields.
Any use of this information is at the reader’s own risk. Open Medscience and its contributors shall not be held liable for any loss, injury, or damage resulting from the use or reliance upon the material published herein. Readers are encouraged to consult original research papers and trusted sources for detailed study or application.
You are here: home » diagnostic medical imaging blog »