Peptide Receptor Radionuclide Therapy (PRRT) has emerged as a promising treatment for neuroendocrine tumours (NETs), offering targeted therapy that specifically addresses the unique characteristics of these malignancies. This article reviews the intricacies of PRRT, examining its mechanisms, clinical efficacy, safety profile, and future directions in the treatment of NETs. The discussion also highlights the historical development of PRRT, its integration into current clinical practice, and its role within the broader spectrum of NET management strategies.
Introduction
Neuroendocrine tumours (NETs) represent a diverse group of neoplasms arising from neuroendocrine system cells. These tumours, which can occur in various organs, including the pancreas, gastrointestinal tract, and lungs, often exhibit indolent growth but possess the potential for malignant transformation. The management of NETs has historically been challenging due to their heterogeneity and the lack of effective systemic therapies. However, the advent of Peptide Receptor Radionuclide Therapy (PRRT) has revolutionised the treatment landscape, offering new hope for patients with advanced and metastatic disease.
Historical Perspective of Peptide Receptor Radionuclide Therapy
The concept of targeted radionuclide therapy can be traced back to the early 20th century when radioactive isotopes were used to treat cancer. However, it was not until the 1990s that Peptide Receptor Radionuclide Therapy became a viable treatment modality for NETs. The discovery of somatostatin receptors on the surface of neuroendocrine cells and the development of somatostatin analogues laid the foundation for PRRT. When labelled with radioactive isotopes, these analogues could selectively bind to somatostatin receptors, delivering targeted radiation to tumour cells while sparing normal tissues.
Mechanisms of Action
PRRT leverages the overexpression of somatostatin receptors, particularly subtype 2 (SSTR2), on the surface of neuroendocrine tumour cells. The therapy involves the administration of a radiolabelled somatostatin analogue, typically lutetium-177 (Lu-177) or yttrium-90 (Y-90), which binds to these receptors. Upon binding, the tumour cell internalises the radiolabelled peptide, allowing the radioactive isotope to deliver a cytotoxic dose of radiation directly to the cell’s DNA, leading to cell death.
The choice of isotope is critical, with Lu-177 being preferred for its relatively short path length and beta emission, which provides effective tumour cell killing with minimal damage to surrounding tissues. Y-90, with its longer path length, may be used in cases where larger tumours or metastases are present, offering the potential for deeper tissue penetration.
Clinical Efficacy of PRRT
The clinical efficacy of Peptide Receptor Radionuclide Therapy has been demonstrated in several pivotal trials, most notably the NETTER-1 trial, which compared Lu-177-DOTATATE (Lutathera) with high-dose octreotide in patients with advanced midgut NETs. The trial results were compelling, showing a significant improvement in progression-free survival (PFS) and overall survival (OS) in the PRRT group. Patients treated with PRRT also experienced a better quality of life and symptom control compared to those receiving conventional therapy.
PRRT has also shown efficacy in other NET subtypes, including pancreatic, bronchial, and paraganglioma/pheochromocytoma tumours. Response rates vary depending on tumour type and prior treatment, but overall, PRRT has demonstrated robust activity in controlling tumour growth and, in some cases, inducing tumour regression.
Safety Profile and Side Effects
While PRRT is generally well tolerated, it is not without side effects. The most common acute side effects include nausea, vomiting, and mild to moderate fatigue. These symptoms are usually transient and manageable with supportive care.
However, long-term toxicities warrant careful monitoring, particularly haematological and renal toxicities. Haematological toxicities, such as thrombocytopenia, anaemia, and leukopenia, are observed in a subset of patients and may necessitate dose adjustments or treatment delays. Renal toxicity, although less common, is a significant concern, particularly with Y-90-based therapies, due to the kidneys’ role in peptide excretion. Strategies to mitigate renal toxicity include amino acid infusion during PRRT to reduce renal radiation dose and careful patient selection and dosing.
Patient Selection and Treatment Protocols
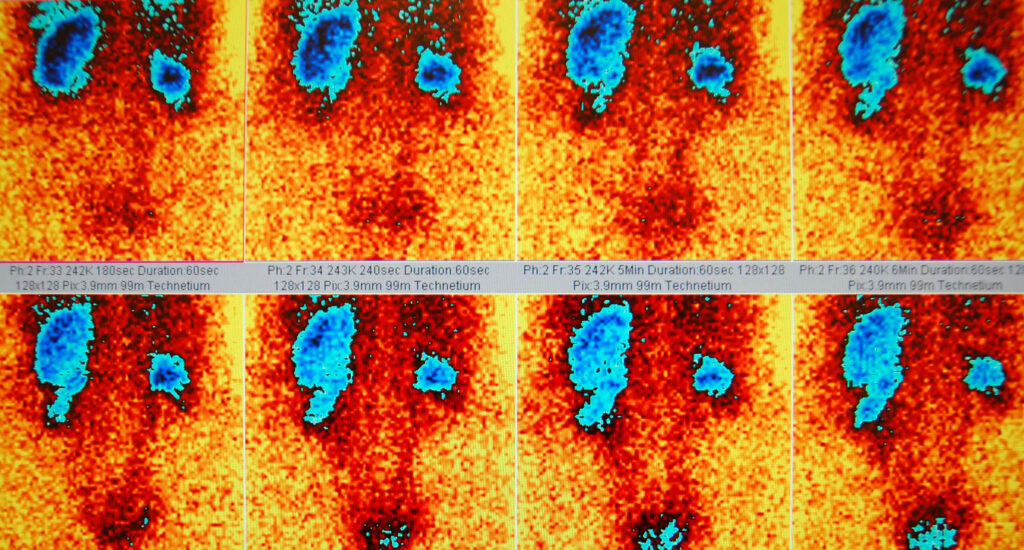
Patient selection is critical to the success of PRRT. Candidates for therapy are typically those with well-differentiated, somatostatin receptor-positive NETs who have progressed on standard therapies or are not candidates for surgical resection. Functional imaging with somatostatin receptor scintigraphy (SRS) or positron emission tomography (PET) using gallium-68 labelled somatostatin analogues is essential for identifying eligible patients.
The treatment protocol for PRRT generally involves the administration of 3 to 4 cycles of radiolabelled peptide, given every 6 to 8 weeks. The dosage and number of cycles may be adjusted based on patient response and tolerance. Follow-up imaging and biochemical markers are used to assess treatment efficacy and guide further management.
Integration with Other Therapies
PRRT is increasingly being integrated into the broader treatment paradigm for NETs, often in combination with other modalities. For example, PRRT can be combined with targeted therapies such as everolimus or sunitinib in selected patients, providing a synergistic approach to tumour control. Additionally, PRRT may be used as a neoadjuvant therapy to downstage tumours before surgical resection or as an adjuvant therapy following surgery to reduce the risk of recurrence.
The combination of PRRT with immunotherapy is also an area of active investigation, with the potential to enhance the anti-tumour immune response and improve outcomes further. While early results are promising, more research is needed to define the optimal combination of strategies and patient populations.
PRRT in Special Populations
The application of PRRT in special populations, such as paediatric patients, those with high tumour burden, and patients with extra-abdominal NETs, presents unique challenges. In paediatric patients, the potential long-term effects of radiation exposure must be carefully weighed against the benefits of treatment. The role of PRRT in high tumour burden settings is still being explored, with considerations around radiation dose and cumulative toxicity.
Patients with extra-abdominal NETs, such as those with bronchial or thymic NETs, may have differing responses to PRRT compared to those with abdominal tumours. These differences may be due to variations in somatostatin receptor expression or tumour biology. Tailoring PRRT to these populations requires a nuanced understanding of the disease and close collaboration with a multidisciplinary team.
Future Directions in PRRT
The future of Peptide Receptor Radionuclide Therapy remains exciting, with ongoing research to optimise and expand its use. One area of focus is the development of novel radionuclides and peptides to enhance tumour targeting and minimise side effects. Alpha-emitting isotopes, such as actinium-225, are being investigated for their potent cytotoxic effects and limited penetration, potentially reducing off-target toxicity.
Another promising direction is the combination of PRRT with radiosensitisers or chemotherapy agents to potentiate the therapeutic effects. Understanding the molecular mechanisms underlying NETs’ resistance to PRRT could also lead to new strategies to overcome this challenge and improve patient outcomes.
Advancements in imaging techniques are also expected to refine patient selection and treatment monitoring. The use of advanced PET imaging with novel tracers may provide more accurate assessments of tumour burden and receptor expression, guiding personalised treatment plans.
Challenges and Limitations
While PRRT represents a significant advancement in NET therapy, several challenges and limitations remain. The cost of PRRT can be prohibitive, limiting access to treatment in some regions. Additionally, the availability of specialised centres equipped to deliver PRRT is limited, which may pose logistical challenges for patients requiring long-distance travel.
Another limitation is the variability in patient response, with some individuals experiencing limited benefit from PRRT. This variability may be due to differences in tumour biology, receptor expression, or previous treatments. Further research is needed to identify predictive biomarkers that can help select patients most likely to benefit from PRRT.
PRRT in the Global Context
The adoption of PRRT varies globally. Some countries have established PRRT as a standard of care for certain NETs, while others are still in the early stages of implementation. Factors influencing this variability include healthcare infrastructure, regulatory approvals, and the availability of radiopharmaceuticals.
PRRT has been widely adopted in Europe, particularly in countries like Germany and the Netherlands, where centres of excellence have been established. The European Neuroendocrine Tumour Society (ENETS) has played a pivotal role in promoting PRRT through guidelines and educational initiatives.
In contrast, access to PRRT in the United States has been more limited, partly due to regulatory hurdles and the relatively recent approval of Lu-177-DOTATATE by the Food and Drug Administration (FDA). Nonetheless, the therapy is gaining traction as more centres develop the capability to offer PRRT and as awareness of its benefits grows.
In other regions, such as Asia and Latin America, the adoption of PRRT is in its nascent stages. Efforts to build capacity, train healthcare professionals, and secure access to radiopharmaceuticals are ongoing, with the potential to expand the availability of this life-saving therapy to a broader patient population.
Patient Perspectives and Quality of Life
For many patients with NETs, PRRT has provided not only a therapeutic benefit but also an improvement in quality of life. Patients often report a reduction in tumour-related symptoms, such as flushing, diarrhoea, and pain, which can significantly enhance their day-to-day functioning and well-being.
Patient perspectives are an essential aspect of evaluating the success of PRRT. Surveys and qualitative studies have shown high levels of patient satisfaction with PRRT, particularly in terms of symptom relief and the perceived effectiveness of the treatment. However, the burden of travel, the frequency of treatments, and the anxiety associated with potential side effects are also important considerations.
Supportive care, including psychological support, nutritional counselling, and management of side effects, is critical to optimising patient outcomes during and after PRRT. Engaging patients in shared decision-making and providing comprehensive information about the benefits and risks of PRRT can empower them to make informed choices about their treatment.
Ethical Considerations and Informed Consent
The ethical considerations surrounding PRRT are multifaceted, particularly regarding informed consent and managing long-term risks. Given the complexity of PRRT and the potential for both acute and delayed toxicities, it is essential that patients receive clear and comprehensive information about the treatment, including its potential benefits, risks, and alternatives.
Informed consent should involve a detailed discussion of the possible outcomes of PRRT, the likelihood of response, and the potential need for additional therapies. Patients should also be informed about the uncertainties associated with long-term outcomes, particularly in terms of secondary malignancies or other radiation-induced complications.
Ethical considerations also extend to equitable access to PRRT. An important goal is to ensure that all eligible patients, regardless of their geographical location or socio-economic status, have access to this therapy. Reducing disparities in access to PRRT may require policy changes, advocacy efforts, and international collaboration.
Economic Impact and Cost-Effectiveness
The economic impact of PRRT is a significant consideration for healthcare systems and patients alike. The cost of PRRT includes the price of the radiopharmaceuticals and the infrastructure required to administer the therapy safely, including specialised facilities and trained personnel.
Despite its cost, PRRT may offer cost-effectiveness advantages over other treatments for NETs, particularly in improving progression-free survival and reducing the need for more extensive interventions. Economic evaluations have suggested that PRRT can be a cost-effective option, especially when considering the long-term benefits of improved symptom control and quality of life.
Healthcare systems must balance the upfront costs of PRRT with the potential for long-term savings, particularly in terms of reduced hospitalisations, fewer surgical interventions, and prolonged survival. Cost-effectiveness analyses are essential to inform healthcare policy and guide decisions about the allocation of resources for PRRT.
Training and Education for Healthcare Professionals
The successful implementation of PRRT requires specialised training and education for healthcare professionals involved in the care of NET patients. This includes nuclear medicine physicians, oncologists, endocrinologists, radiologists, and nursing staff.
Educational initiatives should focus on the principles of PRRT, patient selection, treatment protocols, and the management of side effects. With regular case discussions and tumour board meetings, multidisciplinary collaboration is essential to ensure that PRRT is integrated effectively into patient care.
Ongoing professional development and training opportunities are important to keep healthcare providers updated with the latest advances in PRRT and ensure that patients receive the highest standard of care. This may include attending workshops, conferences, and hands-on training sessions at centres of excellence.
Conclusion
Peptide Receptor Radionuclide Therapy has fundamentally changed the approach to treating neuroendocrine tumours, offering a targeted and effective option for patients with advanced disease. Its ability to improve progression-free survival, overall survival, and quality of life has solidified its role in the management of NETs.
However, the successful application of PRRT requires careful patient selection, a thorough understanding of the therapy’s mechanisms and potential side effects, and a commitment to ongoing research and innovation. As the field of PRRT continues to evolve, it holds the promise of further improving outcomes for patients with neuroendocrine tumours and expanding the horizons of targeted cancer therapy.
Disclaimer
The information provided in this article, Advances in Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumours, is intended for general educational and informational purposes only. It does not constitute medical advice, diagnosis, or treatment, and should not be relied upon as a substitute for consultation with qualified healthcare professionals.
Open Medscience and the article’s authors do not endorse or recommend any specific treatments, procedures, medications, or opinions mentioned herein. Any medical decisions should be made in consultation with a medical practitioner who is familiar with your individual health circumstances and history.
While efforts have been made to ensure the accuracy and timeliness of the information presented, Open Medscience makes no guarantees regarding its completeness or suitability for any purpose. The field of medical science is continuously evolving, and readers are encouraged to consult up-to-date clinical guidelines and evidence-based resources.
Open Medscience assumes no responsibility or liability for any direct or indirect loss or damage resulting from the use or misuse of information contained in this article.
If you are experiencing a medical emergency or have questions about your health, seek immediate advice from a qualified healthcare provider.
home » blog » radiotherapeutics »