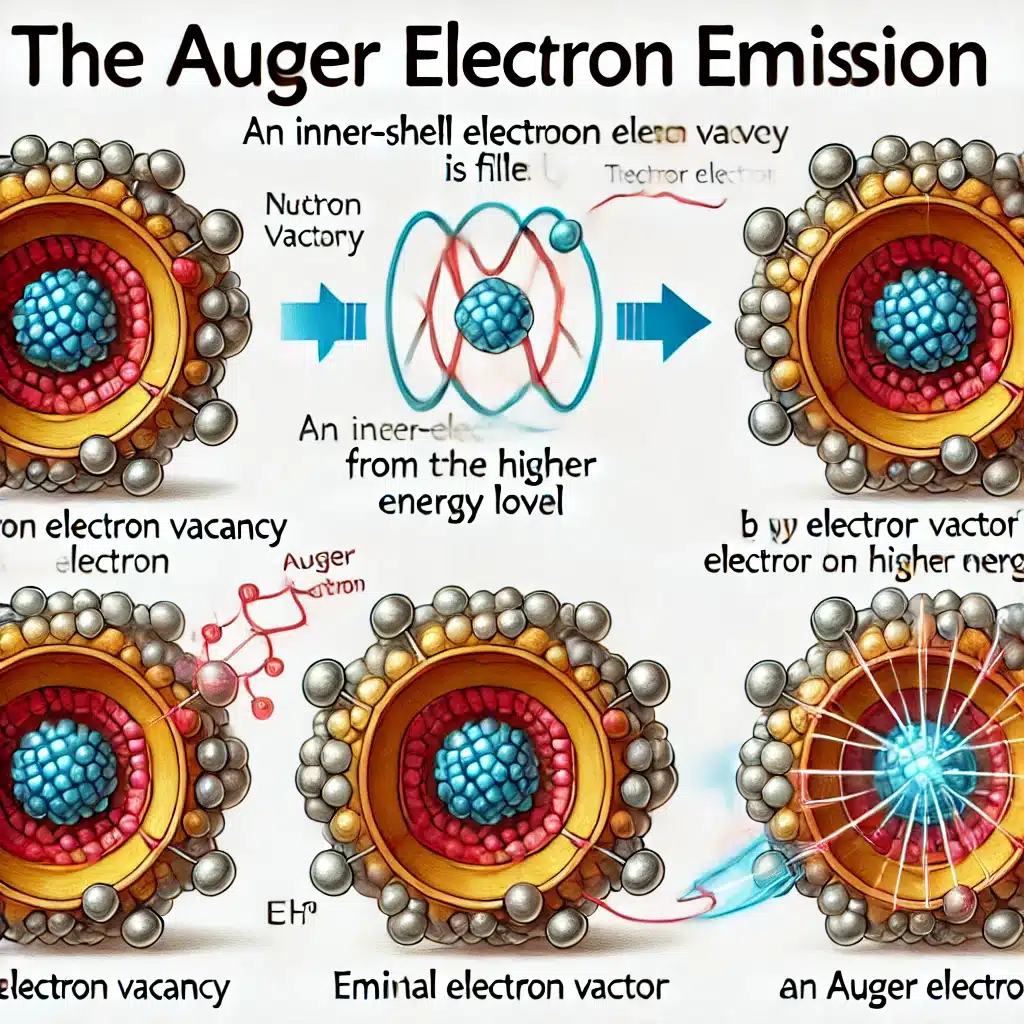
Cancer
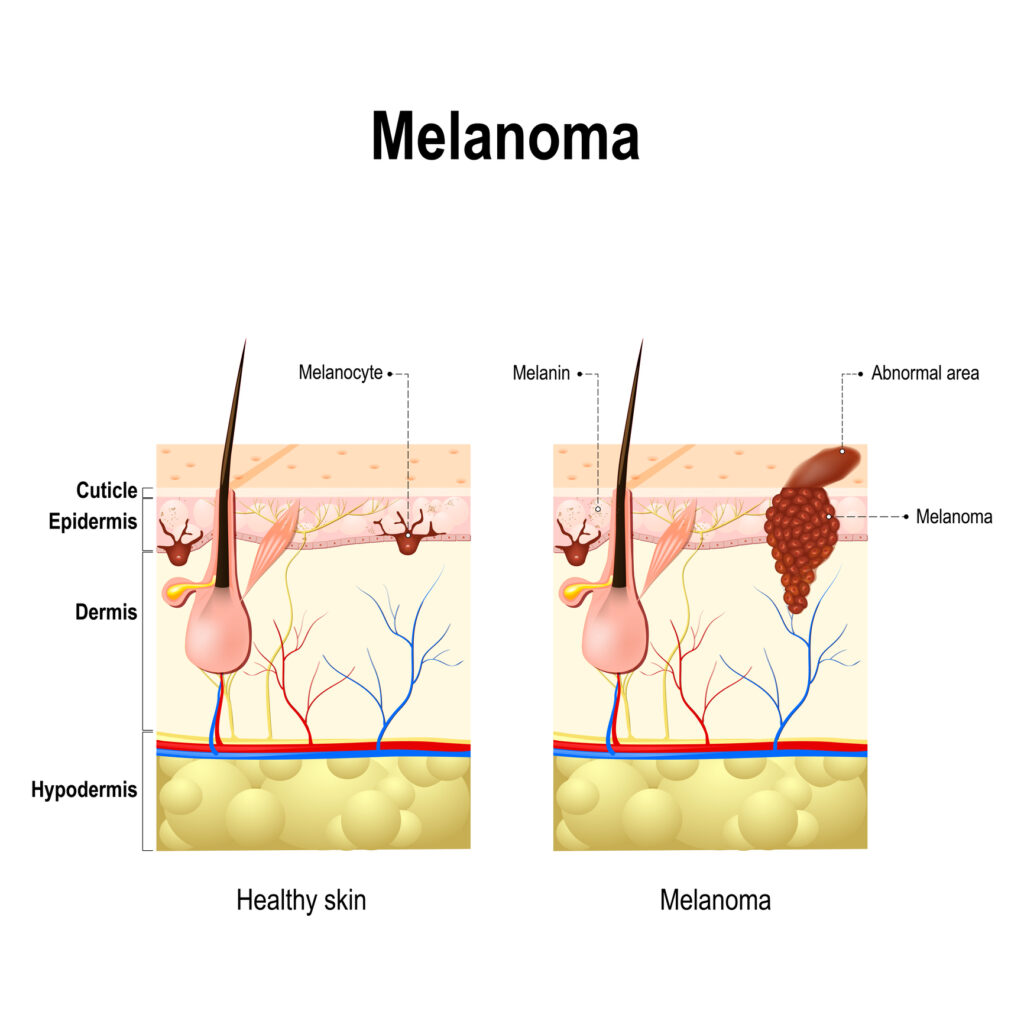
Cancer is a product of cellular changes that cause disease through the uncontrolled growth and division of cells. Over the past 150 years, many hypotheses have been proposed to explain the origin of cancer cells. These include the unregulated proliferation of cancer cells, metastasis and the histological classification of the cancer tissue (benign or malignant tumour).
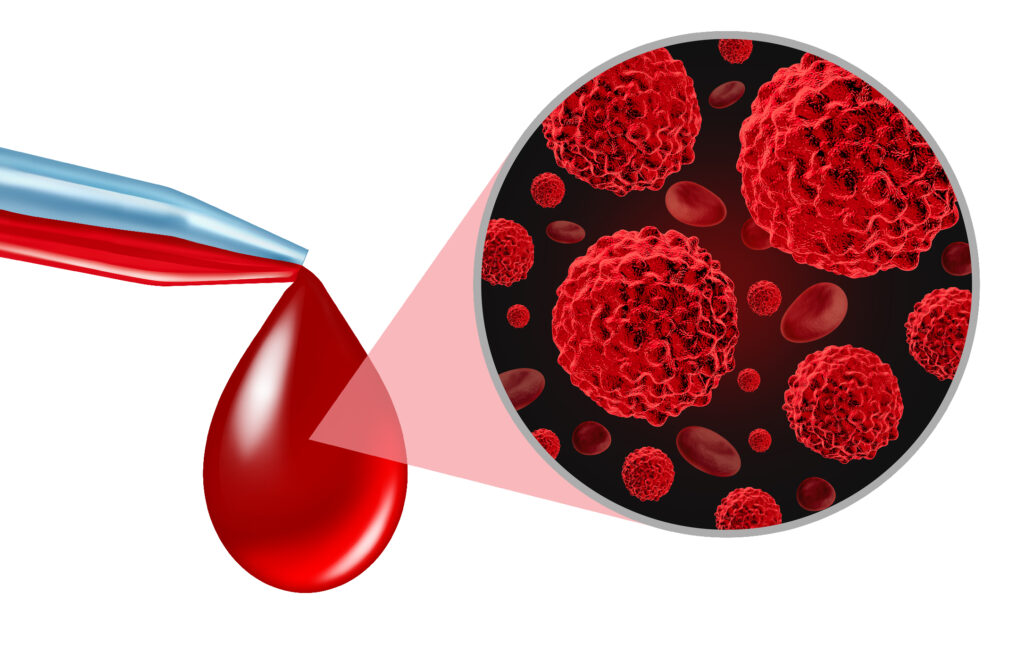
In 1845, Rudolf Virchow found abnormal increases in white blood cells in some patients, and in 1847 identified the condition as a blood disease called leukämie (leukaemia). In 1857, Virchow was the first to identify chordoma, a tumour developed at the skull’s base. Also, in 1858, he presented that cancer cells are the body’s own cells.
Currently, the most accepted theory of cancer is based on the hysteron proteron of the somatic mutation theory (SMT). In this model, the first event (mutations) occurs after the cell has been transformed from a normal cell to a cancer cell via a process termed carcinogenesis. These mutations have increasingly been perceived as the causal event in the origin of the vast majority of cancers.
Currently, this model is challenged by a growing amount of experimental data and arguments that could either not be explained by the model or contradict this model.
One study of three subtypes of ependymoma (glioma) brain tumours found that one subtype carried an intrachromosomal translocation (a segment breaking off the chromosome and rejoining it at a different location), creating a new tumour-driving gene. The second subtype lacked tumour-driving mutations and had epigenetic modifications compared to the third subtype, which had neither gene mutations nor epigenetic aberrations.
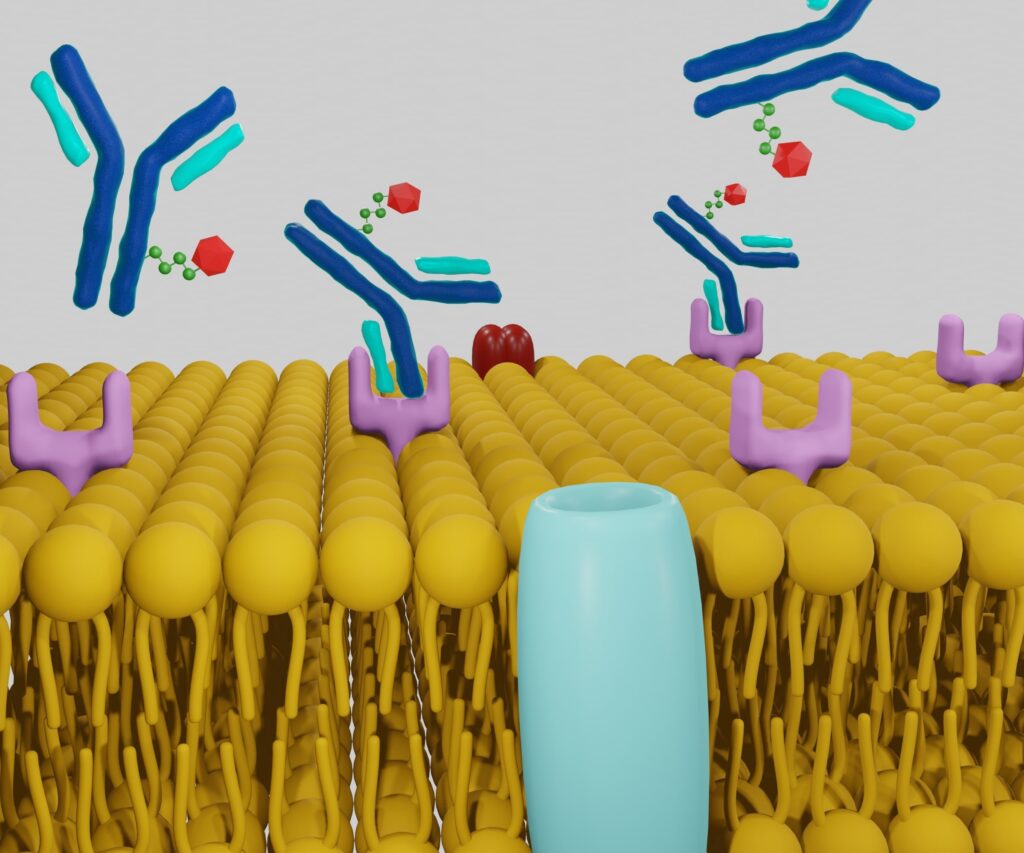
Furthermore, the fact that normal tissues can display massive genetic changes, including changes in cancer-initiating and cancer-driving genes. Over the past few decades, several transfer experiments have demonstrated tumour-suppressing effects on normal cytoplasm. These include mitochondrial transfer, which has the ability to suppress tumour growth. This could be demonstrated – despite the presence of cancerous nuclear genomes – by showing the introduction of non-cancerous mitochondria into highly malignant breast cancer cells. This approach reversed the malignancy and down-regulated several oncogenic pathways, such as invasion and in vivo tumour growth.
Moreover, several non-genotoxic (non-mutagenic) carcinogens, including dichlorobenzene and chloroform, initiate cancer formation. Other oncological theories include oxidative phosphorylation, which results in cellular energy loss and highly impacts cancer formation.
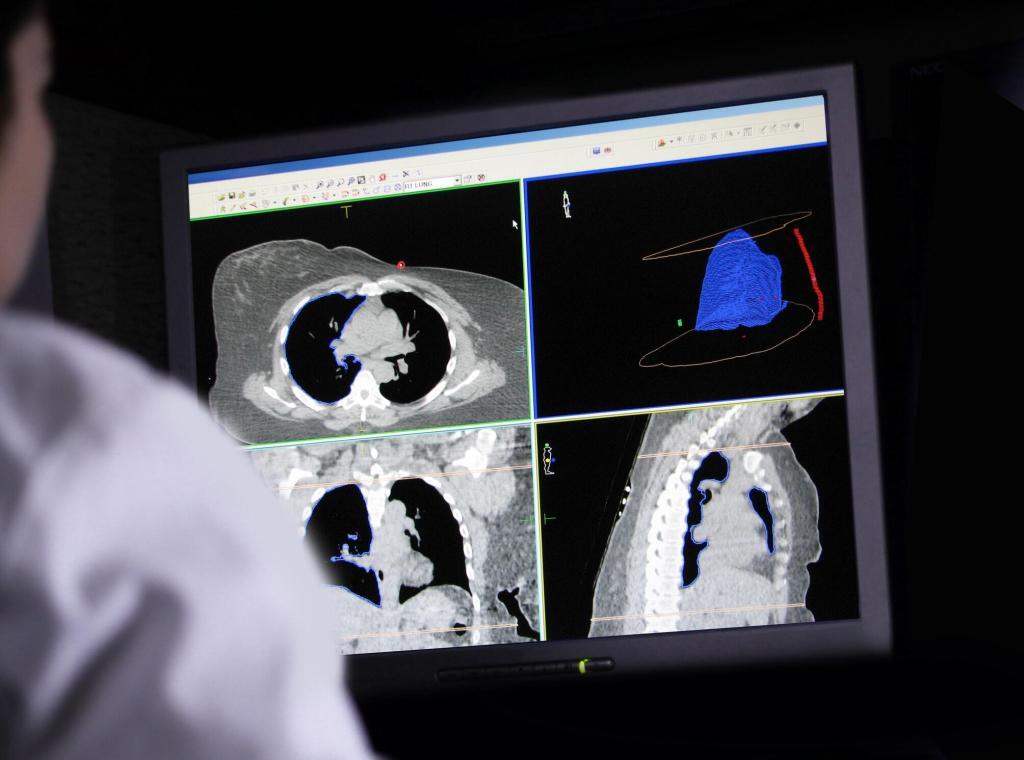
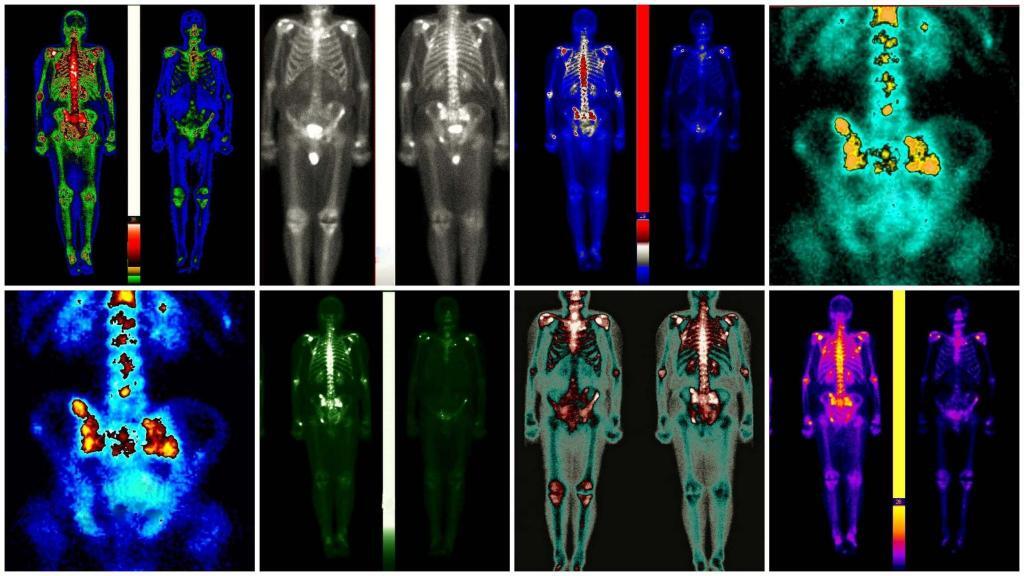
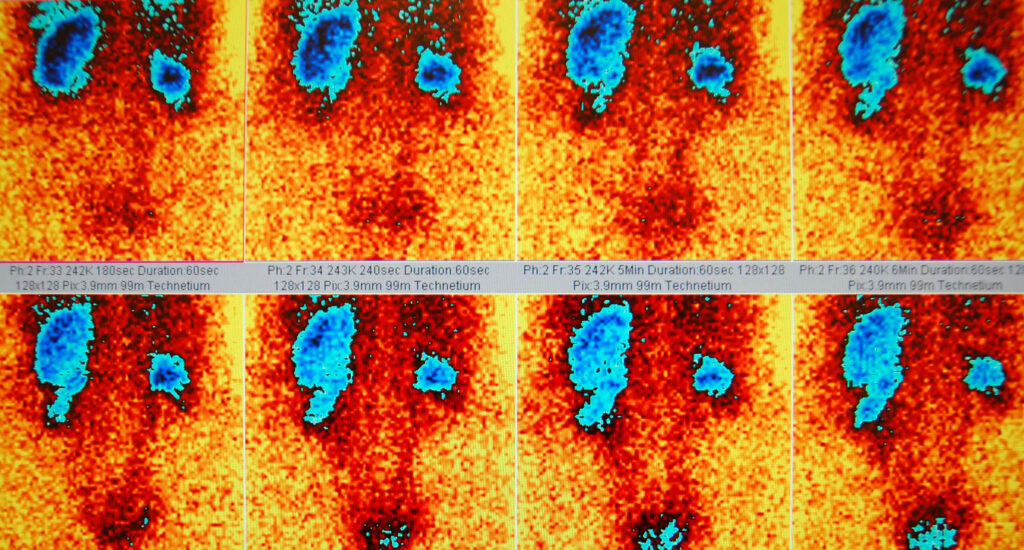
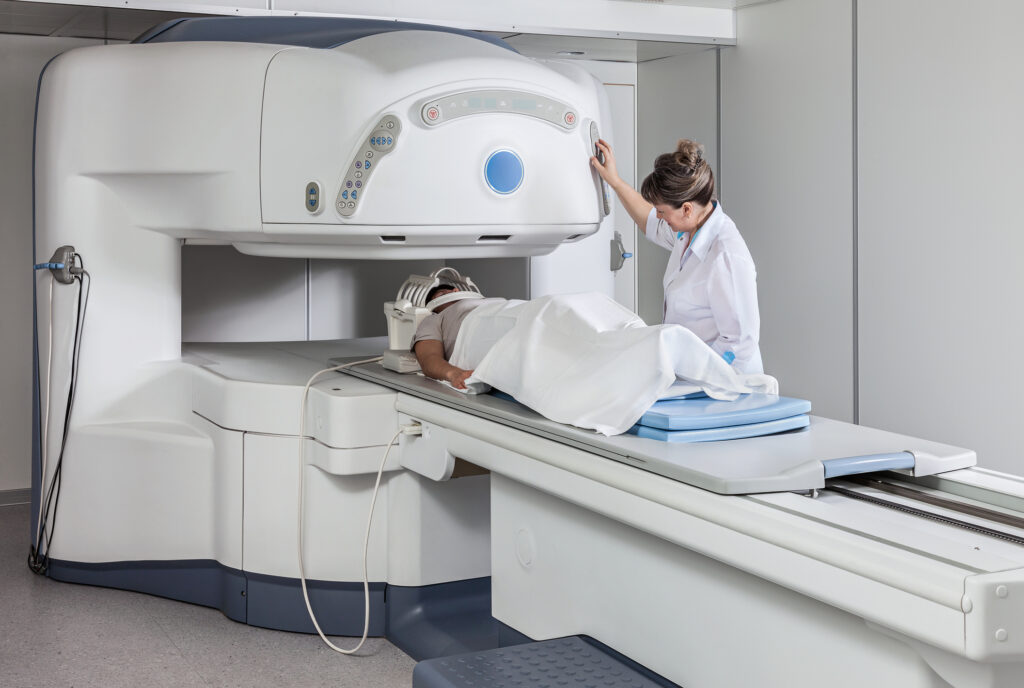
Currently, several imaging modalities are available to clinicians who diagnose, stage and treat human cancer: X-ray (plain film and computed tomography), ultrasound, magnetic resonance imaging, single-photon emission computed tomography, positron emission tomography and optical imaging. Of these, only four (MRI, CT, PET and SPECT) are capable of 3-D detection of cancer anywhere in the human body.
You are here:
home » cancer