This article looks into the crucial role of Accelerated Mass Spectrometry (AMS) in ADME (Absorption, Distribution, Metabolism, and Excretion) studies, particularly in the context of microdosing with carbon-14-labelled compounds. AMS is a highly sensitive technique that has revolutionised the ability to trace isotopic labels, offering unprecedented precision in pharmacokinetic studies at extremely low doses. The integration of AMS with microdosing strategies has led to significant advancements in drug development, allowing for early assessment of a drug’s ADME profile with minimal risk to participants. The article also explores the comparative advantages of AMS over other technologies, its applications in various stages of drug development, and the potential future developments in this field.
Introduction to Accelerated Mass Spectrometry
In the area of pharmaceutical research, understanding a drug’s pharmacokinetic profile is essential for its development and eventual approval for clinical use. This understanding is encapsulated in ADME studies, which assess how a drug is absorbed, distributed, metabolised, and excreted within the body. Traditionally, these studies require relatively high doses of the drug, which can pose ethical and safety challenges, particularly in early-stage human trials. However, the advent of microdosing—where extremely low doses of a drug are administered—has mitigated many of these concerns. Central to the success of microdosing is the use of Accelerated Mass Spectrometry (AMS), a technology that allows for the detection and quantification of isotopic labels, such as carbon-14, at ultra-low concentrations.
The Role of ADME Studies in Drug Development
ADME studies are a cornerstone of drug development, providing critical data on a drug’s behaviour in the human body. These studies inform dosage levels, potential side effects, and the overall efficacy of a drug. Understanding the pharmacokinetics of a drug is not only vital for determining its therapeutic potential but also for ensuring patient safety. ADME studies traditionally involve the administration of labelled compounds to trace the drug’s journey through the body, but these studies typically require relatively large doses of the drug, which can be problematic, especially in early-phase clinical trials.
Challenges in Conventional ADME Studies
Conventional ADME studies, while effective, come with several challenges. High doses required for detection can result in toxicity, particularly in early human trials, posing ethical concerns. Moreover, the process of synthesising and administering labelled compounds can be time-consuming and costly. These limitations have prompted the search for alternative approaches that reduce the dose required while still providing accurate and reliable data.
The Emergence of Microdosing
Microdosing has emerged as a promising solution to some of these challenges. In microdosing, a subtherapeutic dose—typically less than 1% of the dose calculated to produce a pharmacologic effect—is administered to study its pharmacokinetics in humans. This approach minimises the risk of adverse effects while still providing valuable data on the drug’s behaviour in the body. However, the extremely low doses used in microdosing necessitate highly sensitive detection methods, leading to the integration of AMS in these studies.
Accelerated Mass Spectrometry (AMS): An Overview
Accelerated Mass Spectrometry (AMS) is a highly sensitive analytical technique capable of detecting isotopic labels at ultra-trace levels. Originally developed for radiocarbon dating, AMS has been adapted for use in pharmacokinetic studies due to its unparalleled sensitivity, allowing for the detection of carbon-14-labelled compounds at concentrations as low as attomolar levels.
The Working Principle of AMS
AMS works by accelerating ions to extraordinarily high kinetic energies before mass analysis. This process significantly increases the sensitivity and specificity of isotopic detection. In pharmacokinetic studies, AMS is typically used to measure the concentration of carbon-14-labelled compounds in biological samples. Carbon-14 is a radioactive isotope that decays at a known rate, and its presence in a sample can be detected with high precision using AMS, even at very low levels.
Advantages of AMS in ADME Studies
The key advantage of AMS in ADME studies is its sensitivity. This allows researchers to administer microdoses of a drug and still obtain accurate and reliable pharmacokinetic data. AMS can detect isotopic labels in samples as small as a few millilitres, making it ideal for studies where sample availability is limited. Furthermore, AMS is highly specific, reducing the risk of interference from other compounds in the sample.
Comparison with Other Technologies
While other technologies, such as liquid chromatography-mass spectrometry (LC-MS) and positron emission tomography (PET), are also used in ADME studies, they do not offer the same level of sensitivity as AMS. LC-MS, for example, requires larger sample sizes and higher doses of the drug to achieve similar levels of detection, while PET, though highly sensitive, involves the use of positron-emitting isotopes, which have much shorter half-lives than carbon-14. This limits the duration of studies and complicates the logistics of isotope production and administration.
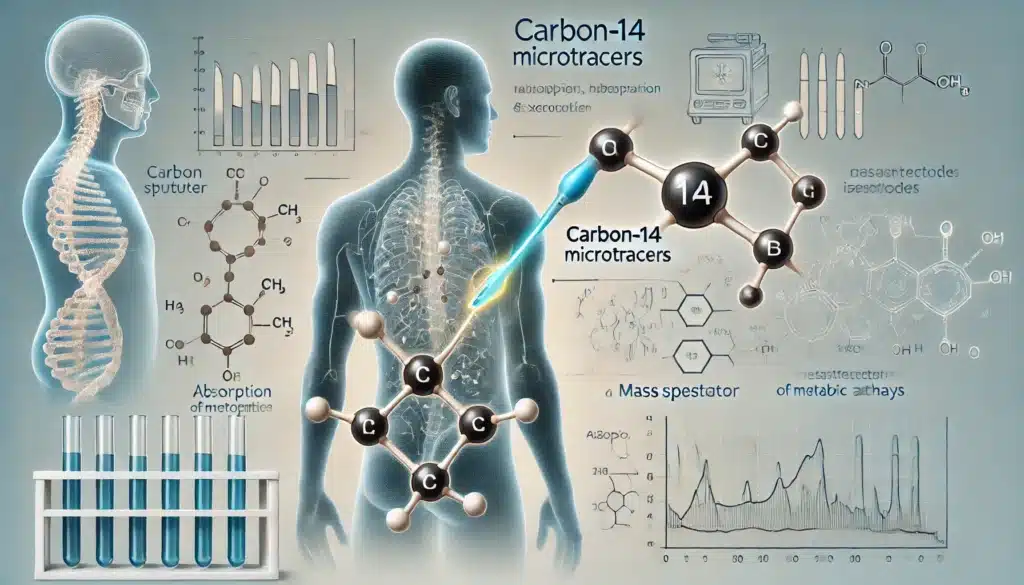
Microdosing with Carbon-14 Compounds
The use of carbon-14-labelled compounds in microdosing studies has been transformative in the field of pharmacokinetics. Carbon-14 has a half-life of approximately 5,730 years, making it ideal for long-term studies, as the isotope remains detectable over extended periods.
The Process of Carbon-14 Labelling
Carbon-14 labelling involves incorporating the radioactive isotope into the molecular structure of the drug. This is usually done through synthetic chemistry techniques, where a specific carbon atom in the drug’s structure is replaced with carbon-14. The labelled drug is then administered to participants, and its distribution and metabolism can be tracked over time using AMS.
Applications in ADME Studies
In ADME studies, carbon-14 labelling allows for the precise tracking of a drug’s absorption, distribution, metabolism, and excretion. By using AMS to measure the concentration of carbon-14 in biological samples, researchers can determine how the drug is processed by the body, including identifying metabolites and understanding the drug’s clearance mechanisms. This information is crucial for assessing the drug’s safety and efficacy, as well as for designing appropriate dosage regimens.
Benefits of Microdosing with Carbon-14 Compounds
Microdosing with carbon-14-labelled compounds offers several benefits. It allows for the collection of pharmacokinetic data at doses that are too low to produce pharmacological effects, reducing the risk to participants. This is particularly important in early-phase clinical trials, where the safety profile of the drug is not yet fully understood. Additionally, microdosing can accelerate the drug development process by providing early insights into a drug’s ADME characteristics, potentially identifying issues before larger, more expensive studies are conducted.
Applications of AMS in Drug Development
AMS has become an invaluable tool in various stages of drug development, from preclinical studies to post-marketing surveillance. Its ability to provide precise pharmacokinetic data at low doses has expanded its use beyond traditional ADME studies.
Preclinical Studies
In preclinical studies, AMS can be used to evaluate the pharmacokinetics of new drug candidates in animal models. By administering microdoses of carbon-14-labelled compounds, researchers can obtain detailed data on the drug’s behaviour in vivo, helping to predict its performance in humans. This can inform decisions about whether to advance a drug candidate to clinical trials, potentially saving time and resources.
Clinical Trials
During clinical trials, AMS is particularly useful in Phase 0 and Phase I studies, where the primary goal is to assess the safety and pharmacokinetics of a drug. Microdosing studies using AMS can provide early pharmacokinetic data, allowing researchers to identify any potential issues with the drug’s ADME profile before progressing to higher doses in later trial phases. This can help to mitigate the risk of adverse effects and improve the overall efficiency of the drug development process.
Post-Marketing Surveillance
Even after a drug has been approved and brought to market, AMS can be used in post-marketing surveillance to monitor its safety and efficacy in the general population. By using AMS to analyse the pharmacokinetics of the drug in real-world settings, researchers can detect any unexpected issues, such as interactions with other medications or differences in drug metabolism among diverse populations.
Technological Advancements and Future Directions
As AMS technology continues to evolve, its applications in pharmacokinetic studies are likely to expand. Emerging developments in AMS instrumentation and data analysis are poised to further enhance the sensitivity and specificity of the technique, making it even more valuable in drug development.
Advances in AMS Instrumentation
Recent advancements in AMS instrumentation have focused on increasing the sensitivity and speed of analysis. Newer AMS systems are capable of detecting even lower concentrations of isotopic labels, reducing the amount of drug required for studies. These systems also offer faster analysis times, allowing for more efficient processing of large numbers of samples. Additionally, improvements in the automation of sample preparation and data analysis are streamlining the workflow, making AMS more accessible to researchers.
Integration with Other Technologies
The integration of AMS with other analytical techniques, such as LC-MS and nuclear magnetic resonance (NMR) spectroscopy, is opening up new possibilities for pharmacokinetic studies. By combining the strengths of these technologies, researchers can obtain a more comprehensive understanding of a drug’s ADME profile. For example, LC-MS can be used to identify and quantify metabolites, while AMS provides precise measurements of the parent drug and its metabolites at ultra-low concentrations.
Future Applications in Personalised Medicine
As the field of personalised medicine continues to grow, AMS is likely to play an increasingly important role in tailoring drug therapies to individual patients. By using AMS to analyse the pharmacokinetics of drugs in specific patient populations, researchers can identify factors that influence drug metabolism, such as genetic variations, age, and underlying health conditions. This information can be used to develop personalised dosing regimens that maximise efficacy while minimising the risk of adverse effects.
Disclaimer
The content of this article is provided for informational and educational purposes only and does not constitute medical, scientific, or regulatory advice. Open Medscience makes no representations or warranties regarding the accuracy, completeness, or suitability of the information contained herein. The techniques, technologies, and applications described, including Accelerated Mass Spectrometry (AMS) and microdosing with carbon-14 compounds, should only be employed by qualified professionals in accordance with applicable ethical standards and regulatory requirements.
Readers are advised to consult appropriate professionals or authorities before acting on any information presented in this article. The views expressed are those of the authors and do not necessarily reflect the official policy or position of Open Medscience or its affiliates.
The inclusion of specific methods or technologies does not imply endorsement by Open Medscience, and the mention of any products or services does not constitute a recommendation or guarantee of efficacy or safety.
home » diagnostic medical imaging blog » careers »