Summary: Electron beam radiotherapy has been a mainstay in clinical oncology for treating superficial tumours, offering precise dose delivery to shallow tissues while sparing deeper organs. The process of planning such treatments is critical to achieving optimal therapeutic outcomes. This blog article explores the essential principles, techniques, advancements, and challenges associated with electron beam treatment planning. It discusses conventional and modern approaches, integration with imaging technologies, dosimetric considerations, and the impact of patient-specific factors on planning strategies.
Keywords: Electron radiotherapy; Treatment planning; Dosimetry; Bolus; Monte Carlo simulation; Beam shaping.
Introduction to Electron Beam Treatment Planning
Electron beam therapy (EBT) utilises high-energy electrons generated by a linear accelerator to treat malignancies located near or at the skin surface. The finite penetration depth of electrons makes them ideal for superficial cancers, such as those of the skin, head and neck, chest wall (following mastectomy), and certain nodal regions. In contrast to photon therapy, electrons have a sharp fall-off in dose beyond their penetration depth, allowing better sparing of underlying healthy tissues.
While the physics of electron interactions is well understood, the complexities involved in dose calculation, field shaping, and tissue heterogeneity require sophisticated planning techniques. Electron treatment planning is as much an art as it is a science, necessitating both experience and precision.
Fundamental Principles of Electron Beam Planning
Electron energy is a critical parameter in treatment planning. The energy determines how deeply the beam penetrates before delivering its maximum dose (the depth of the 90% isodose). Typical clinical energies range from 4 MeV to 20 MeV. Lower energies (4–6 MeV) are used for lesions just below the surface, while higher energies (12–20 MeV) are reserved for deeper-seated targets.
A rough estimation commonly used is that the practical range (in cm) of electrons is approximately one-half their energy in MeV. Therefore, a 10 MeV beam penetrates to a depth of roughly 5 cm.
Dose Distribution Characteristics
Unlike photon beams, electron beams exhibit a relatively uniform dose distribution up to a certain depth, followed by a steep dose fall-off. This profile is advantageous in sparing tissues beyond the target. However, dose uniformity can be disrupted by irregular patient surfaces, air cavities, or tissue inhomogeneities, necessitating tailored planning techniques.
Bolus and Skin Dose Management
A bolus – a tissue-equivalent material – is frequently used in electron therapy to bring the dose closer to the surface or to compensate for irregular contours. The placement and thickness of the bolus are integral to achieving an even dose distribution. Errors in bolus placement or inconsistency in its composition can lead to underdosing or hot spots.
Traditional Planning Techniques
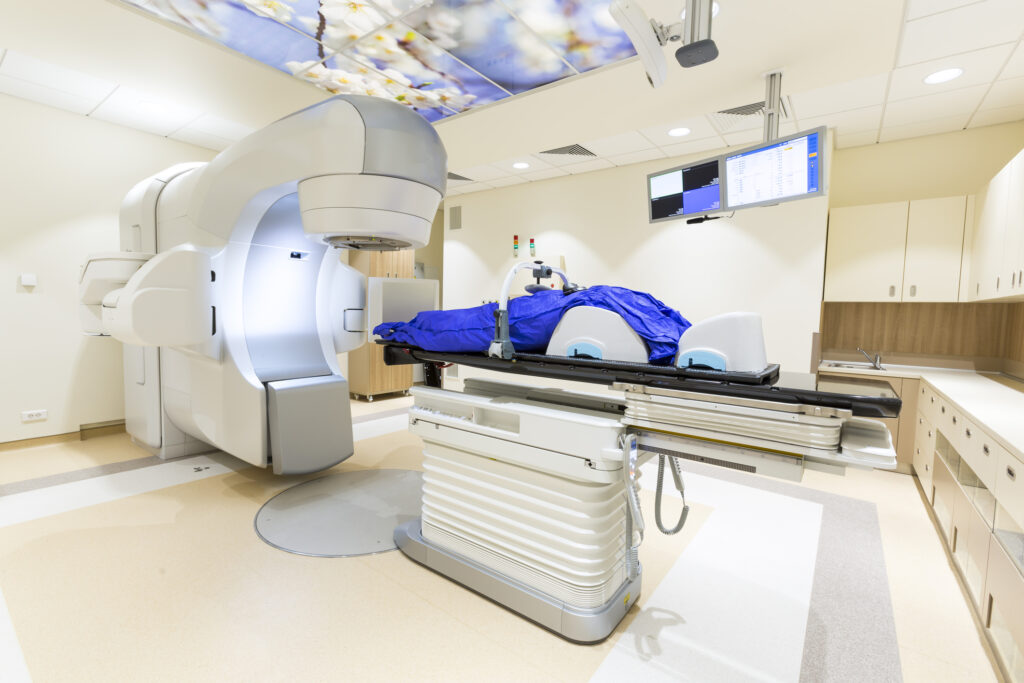
Historically, electron beam planning was performed manually using pre-calculated data such as percentage depth dose (PDD) curves and isodose distributions from water phantoms. The planner would superimpose these curves onto patient contours derived from radiographs or surface measurements.
This approach, while practical, relied heavily on the planner’s experience and offered limited customisation for irregular tumour shapes or complex anatomy. Moreover, it lacked the precision required for dose verification in the presence of tissue heterogeneities.
Field Matching and Abutment
Electron beams are often used in conjunction with photon fields, especially in breast cancer treatments. Field matching techniques, including the use of skin markers and careful alignment of field edges, are essential to avoid dose overlaps or cold spots at junctions.
Feathering techniques, where junction lines are shifted during the course of treatment, help distribute dose inhomogeneities. The challenge lies in maintaining uniform coverage without increasing the dose to surrounding healthy tissues.
Advanced Planning Techniques
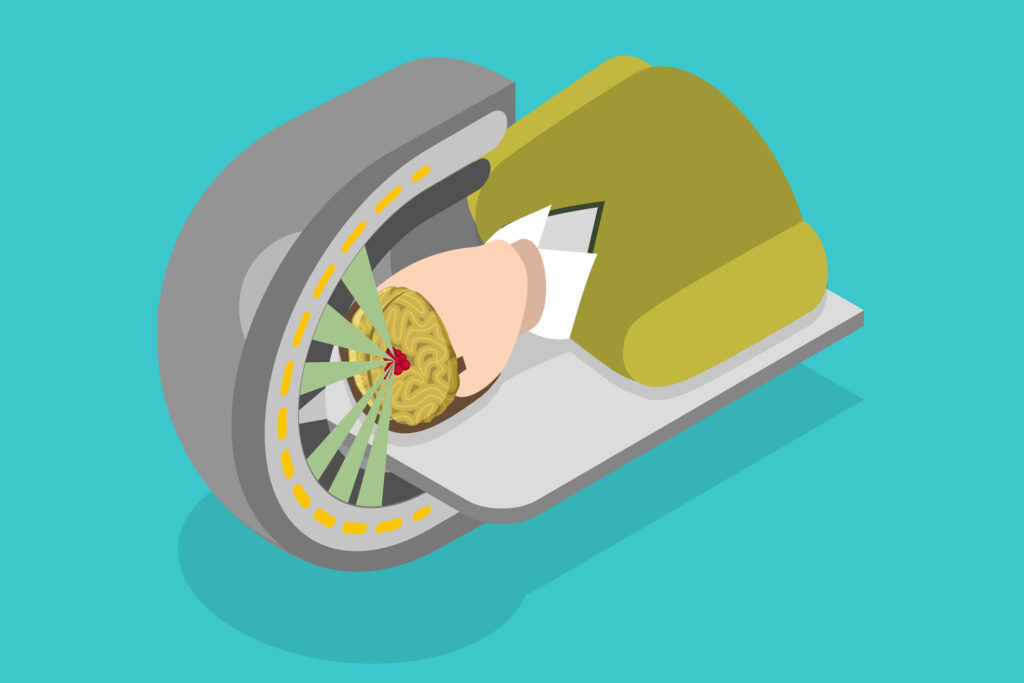
Modern planning systems incorporate three-dimensional (3D) patient imaging data (e.g., CT scans) to build anatomical models. These systems allow dose calculations that account for tissue heterogeneity, surface irregularities, and beam obliquity.
Treatment planning software can simulate the isodose distribution, evaluate dose-volume histograms (DVHs), and adjust field parameters such as energy, beam modifiers, and bolus. Sophisticated contouring tools also permit more precise targeting and sparing of normal tissue.
Custom Bolus Design
With 3D printing technology and imaging data, patient-specific bolus can be fabricated to conform precisely to irregular surfaces. This improves skin contact and reduces air gaps, leading to better dosimetric outcomes.
Material density and thickness can be controlled to modulate dose intensity, making it especially useful in treating curved or uneven anatomical sites, such as the nose, ear, or scalp. Several centres have begun integrating custom bolus workflows into routine practice.
Beam Modifiers and Cutouts
Electron beam shapes are typically defined by custom-made cutouts fitted into the applicator. These cutouts are created using low-melting alloys such as Cerrobend. The precision of the cutout design directly affects the conformity of the treatment.
Advanced techniques may employ bolus-shaping or intensity-modulated bolus layers to further refine beam shaping. In some institutions, multi-leaf collimators (MLCs) are being explored for use with electron beams, although they are more commonly used in photon therapy.
Monte Carlo and Model-Based Algorithms
Conventional planning systems often use pencil beam or simplified convolution/superposition algorithms, which may not accurately predict dose in heterogeneous media. Their limitations become evident in the head and neck or thoracic regions where air cavities, bone, and soft tissues coexist.
Monte Carlo Simulation
Monte Carlo (MC) algorithms offer a gold-standard solution for electron dose calculation. By modelling the transport and interaction of individual electrons, MC simulations achieve unparalleled accuracy. They consider stochastic variations, scattering effects, and tissue heterogeneities with high precision.
Although previously restricted by long computation times, advances in hardware and algorithm optimisation have enabled Monte Carlo-based planning to become clinically viable. Some treatment planning systems now incorporate MC engines as part of their standard package.
Clinical Integration
MC-based planning is particularly advantageous in areas with sharp dose gradients or complex geometries. Clinical studies have shown improved target coverage and reduced normal tissue dose when MC algorithms are employed. The challenge lies in integrating these into routine clinical workflows without significantly increasing planning time.
Challenges and Future Directions
Changes in patient anatomy between and during treatments due to weight loss, swelling, or movement can impact electron beam accuracy. Unlike photon IMRT, electron therapy has less adaptability, and ongoing research is examining adaptive strategies, including real-time imaging and deformable dose accumulation.
Integration with Image Guidance
While image-guided radiotherapy (IGRT) is well-established for photons, its application in electron therapy remains under development. Innovations in surface-guided radiotherapy (SGRT) and cone-beam computed tomography (CBCT) are being gradually adapted for electron workflows, enabling better verification of setup and target position.
Electron Arc Therapy and Emerging Modalities
Techniques such as modulated electron radiation therapy (MERT) and electron arc therapy are being investigated. These promise improved dose conformity and coverage for complex targets. However, they require highly advanced planning systems and strict quality assurance procedures.
Additionally, the emergence of FLASH radiotherapy – ultra-high dose rate electron therapy – has sparked interest in its potential to spare normal tissue while maintaining tumour control. Its planning demands are currently being explored in preclinical and early clinical trials.
Conclusion
Electron beam treatment planning continues to evolve with technological progress and growing clinical experience. From manual methods and clinical templates to advanced 3D planning and Monte Carlo simulations, the planning process has become more precise, patient-specific, and outcome-driven.
Success in electron therapy depends on the thoughtful selection of beam parameters, the appropriate use of bolus and modifiers, and the ability to adapt to anatomical variations. As imaging, computing, and delivery technologies mature, electron beam therapy is expected to retain its relevance and expand into more sophisticated applications.
For clinical physicists and radiation oncologists, mastering these planning techniques is essential not only for current practice but also for embracing future innovations in the treatment of superficial cancers.
Disclaimer
The content presented in this article is intended for informational and educational purposes only. Open Medscience does not provide medical, clinical, or professional advice, and the information herein should not be interpreted as such. While every effort has been made to ensure the accuracy of the content at the time of publication, advances in research and clinical practice may lead to changes in protocols or recommendations.
Clinicians, medical physicists, and other healthcare professionals should exercise their own clinical judgement and consult appropriate sources before applying any of the techniques or information discussed. The authors and Open Medscience accept no liability for any errors, omissions, or consequences arising from the use of this material.
This article does not replace professional training or institutional guidelines, and readers are encouraged to refer to their local protocols and regulatory requirements.
You are here: home » diagnostic medical imaging blog »