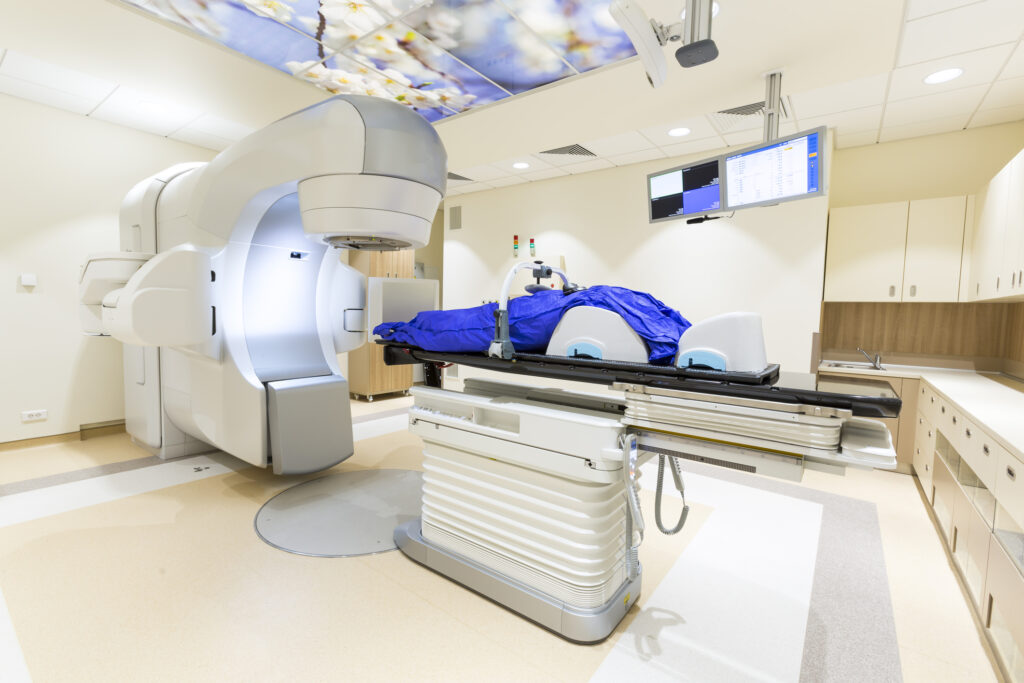
These cancer-destroying machines can provide proton beam therapy via pencil beam scanning.
Proton beam therapy – a radiotherapy revolution
In July 2018, Simon Hardacre was the first person in the UK to receive high-energy proton beam therapy to treat an aggressive form of prostate cancer at the Rutherford Cancer Centre in Newport, South Wales. This is the only clinic in the UK where high-energy proton beam therapy is available.
Proton beam therapy in the UK has joined the radiotherapy revolution to fight cancer. This new approach is an advanced form of external radiation therapy which uses high-energy protons rather than photons. The Rutherford Cancer Centre operates proton beam therapy delivered by the IBA Proteus®ONE machine in the UK.
The Proteus®ONE is IBA’s (Ion Beam Applications S.A.) compact single-room proton therapy solution that can be integrated into the healthcare setting. It is smaller and more affordable than conventional multi-room proton systems, which makes it more accessible to clinical institutions worldwide for the treatment of cancer patients. It uses Pencil Beam Scanning technology to minimise radiation exposure to healthy tissue.
Pencil Beam Scanning Proton Therapy is capable of enhancing accuracy by using an ultra-narrow proton radiation beam.
Therefore, it reduces radiation exposure to the organs and healthy surrounding tissue and lowers the associated risk of side effects.
Also, it increases the types of tumours which can be treated with proton therapy to include irregularly shaped tumours which are intertwined with critical tissue and organs.
These cancer-destroying machines can provide proton beam therapy via pencil beam scanning. Consequently, these advances in technology allow the radiation dose to be given to the exact shape of the tumour target volume. During the targeting of the tumour, the patient is positioned on a couch with the most up-to-date imaging capability, which includes cone beam computed tomography allowing the delivery of precise proton beam treatments.
Proton beam therapy is changing the way we manage tumours without causing damage to organs through the technological revolution in radiotherapy, guided imaging systems and treatment planning.
The patient in receipt of proton beam therapy will have a personalised treatment plan according to their own particular needs.
How does proton therapy work?
Conventional radiotherapy uses high-energy X-rays, which damage DNA within the cancer cells. This process causes the cancer cells to undergo apoptosis leading to cell death. Proton beam therapy can also be used following surgery or after chemotherapy, hormone therapy and targeted drug therapy. The advantage of proton beam therapy is that the proton beams can be targeted to the tumour site and, therefore, limit damage to healthy surrounding tissues: this compares more favourably to conventional radiotherapy. The proton beams can be used to treat complex tumours, which are difficult to remove by surgery due to their proximity to sensitive healthy tissues.
The protons are accelerated to 60% of the speed of light – with a kinetic energy of 250 MeV using cyclotrons and synchrotrons – to penetrate approximately 38 cm into the body. During their journey towards the cancer site, small amounts of energy are transferred to the molecular electron clouds causing a low degree of ionisation.
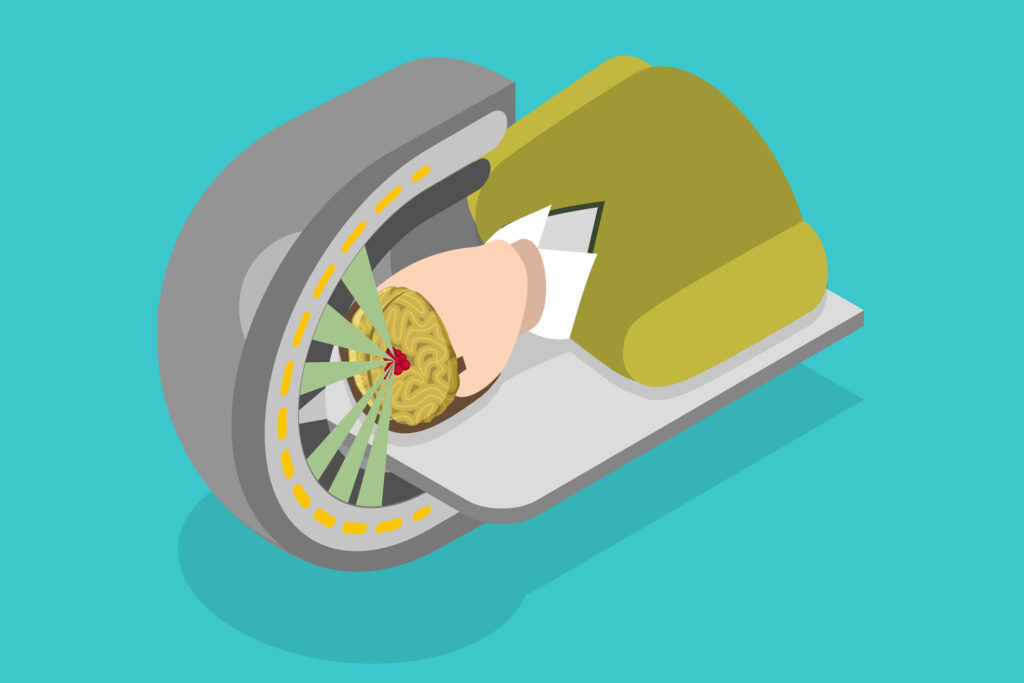
This process slows the mono-energetic ions down, generating a greater linear energy transfer which results in the braking effect of the proton. At this point, there forms an energy burst known as the Bragg peak towards the end of the proton path. In contrast to X-rays, the proton radiation is deposited at a lower dose in front of the tumour. Consequently, the tissue mass behind the tumour is not exposed to radiation. Therefore, this physical phenomenon makes it possible to determine the depth of the Bragg peak, which considers the modulation of the particle velocity and can focus the radiation within the tumour volume. This process is conducted with absolute precision and therefore improves the ratio of therapeutic radiation to the effects of harmful radiation.
The spreading and shaping are achieved electro-mechanically to treat patients with passively-scattered proton therapy. However, a technique that uses magnetic scanning with thin beamlets of protons contains a sequence of energies. Furthermore, the most powerful proton modality is the magnetic scanning technique to treat patients with optimised intensity modulated proton therapy (IMPT).
Applying proton beams instead of X-rays allows medical physicists to increase the therapeutic dose while controlling the dose deposited in healthy tissue. This approach has reduced the radiation deposited in healthy tissue from 43% to 78%, depending on the tumour’s shape.
It is planned for the next three years that the UK will have at least six proton beam centres. However, the UK National Health Service will operate two proton centres, and Proton Partners International will control the remainder. This proton beam therapy revolution will enable first-time cancer patients to be treated in the UK. Before this significant revolution, these same patients would have had to travel to clinics in the US and Europe.
It has been predicted that Proton Partners International will reach approximately 6% of cancer patients who receive radiotherapy every year in the UK.
It is also planned to open more proton therapy centres in Northumberland, Reading, Liverpool and London. The aim is to build eight centres in the UK to be branded as ‘Rutherford Cancer Centres’.
By the end of 2020, the NHS will operate higher capacity proton beam centres at the Christie Hospital in Manchester and University College London Hospital.
Proton beam therapy systems used in the clinical setting
Brief History of Proton Beam Therapy
Proton beam therapy has been driven by cyclotron technology since it was first used in 1932 by Ernest Lawrence at the University of California, Berkeley. This was followed in 1937 by the European cyclotron at the Radium Institute, Leningrad and developed by George Gamow and Lev Mysovskii. However, the largest cyclotron is located at the RIKEN laboratory in Japan and is known as the Superconducting Ring Cyclotron (SRC). The SRC weighs about 8,300 tonnes and accelerates uranium ions to 345 MeV per atomic mass unit. The TRIUMF cyclotron based in Canada can produce a field of 0.46 T while a 23 MHz 94 kV electric field accelerates the 300 μA beam.
Proton beam therapy can trace its progress back to the cyclotron physicist Robert Wilson who in 1946 predicted the use of energetic protons in radiotherapy. In the past two decades, proton therapy has become an emerging treatment option for patients with certain types of cancer as there are advantages over conventional x-ray therapy.
In 1985, at the Fermilab conference, James Slater, Leon Lederman, and Philip Livdahl presented the viability of building a hospital-based proton cyclotron. This was endorsed by the interest of Robert Wilson in the medical use of protons for cancer treatment.
Proton beam therapy uses a beam of energetic protons, while x-rays use a photon to irradiate diseased tissue. The protons and photons must travel through the patient’s skin and surrounding tissues to reach the tumour volume. Photons have no associated mass or charge and can deliver a dose of radiation by penetrating the tissue. This radiation is delivered to a depth of 0.5 cm to 3 cm from the patient’s skin and depends on the initial energy. The energy of the photons diminishes at the tumour target because the radiation ionises the surrounding healthy tissues and the tumour site. Also, the photons leave the patient’s body and continue to emit radiation, known as the exit dose.
However, the proton is heavy (equivalent to ~2000 electrons), and as a charged particle, it gradually loses its speed as it interacts with human tissue. This property gives an element of control and delivers a maximum dose at a precise depth: this is associated with the amount of energy obtained from the cyclotron and is capable of a penetration depth of 38 cm. The advantage of using protons over photons is that protons are very fast at the point of entry into the body and therefore deposit only a small dose on the surrounding tissues.
The beam of protons allows the absorbed dose to increase gradually with greater depth and lower speed. This then produces a peak when the proton comes to rest, known as the Bragg peak. Proton therapy aims to control the proton beam by directing the Bragg peak inside the tumour volume, followed by a burst of energy when the proton suddenly rests during irradiation. The advantage of proton therapy is that it is possible to target tumours within the body and precisely localise the radiation dose with limited damage to the surrounding healthy tissue.
In 1954, the first proton therapy clinic opened at Berkeley treating 30 patients: since 1990, over 17500 patients have been treated. During the development of proton therapy, new guided imaging systems and treatment planning approaches had to be discovered and implemented.
Since the 1970s, proton therapy has been part of the clinical setting in the USA, receiving U.S. Food and Drug Administration approval in 1988. About 100,000 people have received proton therapy at centres throughout Europe, Asia, and the USA.
A breakthrough in cancer diagnosis was the development of X-ray computed tomography (CT) in 1970. These imaging machines could generate 3-D scans to provide tumour volume location and the assignment of CT Hounsfield numbers. This information was beneficial for calculating the electron-density distribution required to perform 3-D dose calculations. The development of CT scanning provides the way forward to CT-based radiation technologies in the treatment planning of cancer patients. Subsequently, in the 1980s, the diagnostic tool magnetic resonance imaging (MRI) arrived, which was central to evaluating the disease states in patients.
The primary emphasis of developing diagnostic imaging tools is finding a relationship between a tumour and surrounding healthy tissue. The advantage of MRI over CT is that there is a generation of images with higher spatial resolution and contrast.
During the 1990s, positron emission tomography (PET) imaging joined the diagnostic toolbox in the treatment planning of cancer patients. Then further advancements led to hybrid scanning machines such as PET-CT, which could visualise tumours and provide more detailed images. PET imaging focuses on distinguishing between tumours and normal tissue sites due to their different metabolism rates.
These modern imaging modalities – in conjunction with proton beam therapy – are used to detect the geometric contours of tumours and to ensure that the correct radiation dose is delivered accurately to the tumour site. Also, further advancements in imaging-guided therapy have produced 4-D computed tomography imaging as the basis for respiratory-gated proton beam therapy to limit motion artefacts.
Today, the apex of medical imaging has combined X-ray radiation techniques with proton beam therapy to treat a broad range of cancers and eradicate them. Cancer treatment depends on the physicist’s ability to conform the irradiation outline to the tumour volume.
The problem arises when conventional radiotherapy techniques introduce a high risk of damaging surrounding healthy tissues. Therefore, treatment planning is important to reduce radiation dosage, limit damage to the surrounding healthy tissues, and limit other side effects.
Prostate cancer
According to Cancer Research UK, there are about 14.1 million new cancer cases globally. The top four cancers are lung, breast, bowel, and prostate. Prostate cancer is caused by the uncontrollable growth of cells in the prostate gland. The prostate neoplasm is the most common form of non-skin cancer in men. Nearly 3% of individuals affected die from this disease due to many medical interventions offered to treat prostate cancer.
The application of proton therapy to treat patients with prostate cancer has increased more than 2-fold from 2004 to 2012. Various studies have shown that the rate of proton beam therapy use has risen from 2.3% in 2004 to 5.2% in 2011 and 4.8% in 2012.
Comparison of treatments for prostate cancer
The overall advantage of proton beam therapy over conventional radiotherapy is the ability to use protons at a higher radiation dosage.
Medical physicists use the emerging concept of theranostics (therapy + diagnostics) to control and manage cancer with the aim of reducing damage to healthy tissue and surrounding organs. Proton beam therapy offers a personalised approach to using radiotherapy to treat cancer.
Advantages of PBT
- Proton beam therapy can precisely target tumours and reduce the exit dose.
- The overall radiation dose in the patient can be lowered.
- The proton beam reduces the side effects of radiation on the surrounding healthy tissues and organs.
- Proton beam therapy is able to deliver an optimal radiation dose to the tumour volume.
- Also, proton beam therapy can be used to treat recurring tumours.
- The quality of life after treatment is significant.
- The long-term and survival rates of using proton beam therapy benefit many tumours.
Other PBT centres
Proton beam therapy systems are available in at least 24 sites across the United States; this includes a new proton therapy centre at the Miami Cancer Institute. These facilities can cost over $225 million each and have been called the single most expensive medical device ever built. Consequently, treatment costs exceed those of traditional photon external-beam radiotherapy (EBRT) modalities.
The conventional linear accelerator (LINAC) produces radiation by accelerating electrons down a long, straight tube. However, protons require much more energy to cause them to move: this can only be achieved using a cyclotron. Cyclotrons can cost up to £100m, and since the advancement of cyclotron technology can be purchased for £25m compared to a conventional radiotherapy machine which costs about £2.5m.
Nevertheless, proton beam therapy is more expensive than conventional LINAC-based radiotherapy and is currently mostly channelled towards paediatric cancers of the brain and spinal cord. In addition, proton beam therapy treatment for the lung and prostate reduces long-term side effects. Also, in patients where the anatomy of a tumour contains critical normal tissues, a dose distribution with protons is the favourable option.
Presently, two proton beam therapy machines are installed at University College Hospital in London and Christie Hospital, in Manchester. These will be equipped with large machines which can cost about £80m and require up to 80 staff to manage each machine.
However, the compact models installed by private sector providers for NHS use cost less than £25m and require only 20 staff. That means the cost per single treatment will be far more expensive in the NHS installations.
Therefore, the UCLH/Christie true cost per treatment will approach £5,000, whereas the cost of the compact model will be less than £1,500.
Radiotherapy in the UK urgently requires upgrades since routine radiation machines are over 10 years old. Replacing these machines with modern versions involves an injection of capital, and by 2020 the NHS may produce a £20bn deficit.
Due to an ever-ageing population, there is a requirement for more effective means of delivering the radiation dose to destroy cancer. Therefore, partnerships with the private sector can help the NHS in the area of proton beam therapy. This is by creating advanced radiotherapy networks throughout the UK which use high-energy beams of protons rather than high-energy X-rays to deliver radiotherapy.
The national proton beam therapy service was integral to the Government’s Cancer Strategy Improving Outcomes: A Strategy for Cancer (2011).
Particle beam therapy facilities in clinical operation (since February 2019)
Particle beam therapy facilities under construction (January 2019)
According to the Particle Therapy Co-Operative Group (PTCOG), at the end of 2017, nearly 200,000 patients were treated globally using particle radiotherapy. This includes 170,500 using protons, 25,700 with carbon-ion radiotherapy, and 3,500 helium ion beams, pions and other particles.
Conclusion
Currently, proton beam therapy is becoming a more accessible radiotherapy treatment. This is mainly due to the ability of protons to deposit a high radiation dose at the cancer site and limit damage to surrounding healthy tissue. The efficacy of using different radiation modalities to create biological effects in cells by targeting with photons (X-rays) and charged particles (protons) is the radiation weighting factor used to calculate the relative biological effectiveness (RBE).
This is defined as the rate of the doses required by two different radiations to cause the same effect level. When calculating the radiation dose, it is essential that the proton RBE generic value is given a more realistic value regarding the conversion of photon dose to proton dose and is dependent on several factors.
Incidentally, the generic value of 1.1 is used in the clinical setting for protons. Currently, there is uncertainty on this dose-weighting factor which is why there is an unfortunate contribution to normal tissue toxicity.
It has been suggested that the proton RBE should be greater than 1.1 and also that it may vary depending on the position relative to the Bragg peak and the increased linear energy transfer. The uncertainty surrounding the proton RBE value may lead to an undesired increase in the radiation dose: for example, to organs most at risk, such as the brainstem, temporal lobe and optic chiasm, if within the vicinity of the tumour volume. Therefore, to reduce the risk of potential radiation toxicity during proton beam therapy, a more rational approach to assigning RBE values is required. These values must consider the dose per fraction and include an assessment of the tissue type and radiobiological tumour characteristics.
Disclaimer
The information presented in this article is intended for educational and informational purposes only. It does not constitute medical advice, diagnosis or treatment and should not be relied upon as such. While every effort has been made to ensure the accuracy of the information at the time of publication (19 February 2019), medical knowledge, technology and clinical practice continue to evolve.
Open Medscience does not endorse or recommend any specific treatment, therapy, product, service, or healthcare provider. Readers are strongly advised to consult a qualified medical professional regarding any questions or concerns they may have about their health, treatment options, or the suitability of proton beam therapy for their individual condition.
Any references to individuals, clinics, technologies, or outcomes are provided for context and should not be interpreted as guarantees of effectiveness or safety. Open Medscience accepts no responsibility or liability for any loss, damage or injury arising from the use of this information.
You are here: home » diagnostic medical imaging blog »