Summary: The recent approval of gozetotide (also known as 68Ga-PSMA-11) marks a significant advancement in the diagnostic landscape of prostate cancer. As a novel PET imaging agent targeting prostate-specific membrane antigen (PSMA), gozetotide enables more accurate detection and staging of prostate cancer than traditional imaging techniques. This article explores the mechanism of action, clinical benefits, regulatory approval, and the implications for patient care in the UK and globally.
Keywords: gozetotide, prostate cancer, PSMA PET, medical imaging, 68Ga-PSMA-11, cancer diagnosis
Introduction: A Breakthrough in Prostate Cancer Imaging
Prostate cancer is the most common cancer among men in the United Kingdom, with around 52,000 new cases diagnosed annually. Early and accurate detection plays a crucial role in determining prognosis and tailoring treatment. While conventional imaging methods such as CT, MRI, and bone scintigraphy have long been the mainstays of prostate cancer staging, their limitations in sensitivity and specificity have often left clinicians with incomplete diagnostic pictures. The recent regulatory approval of gozetotide represents a leap forward in prostate cancer imaging, offering improved accuracy through targeted positron emission tomography (PET).
What is Gozetotide?
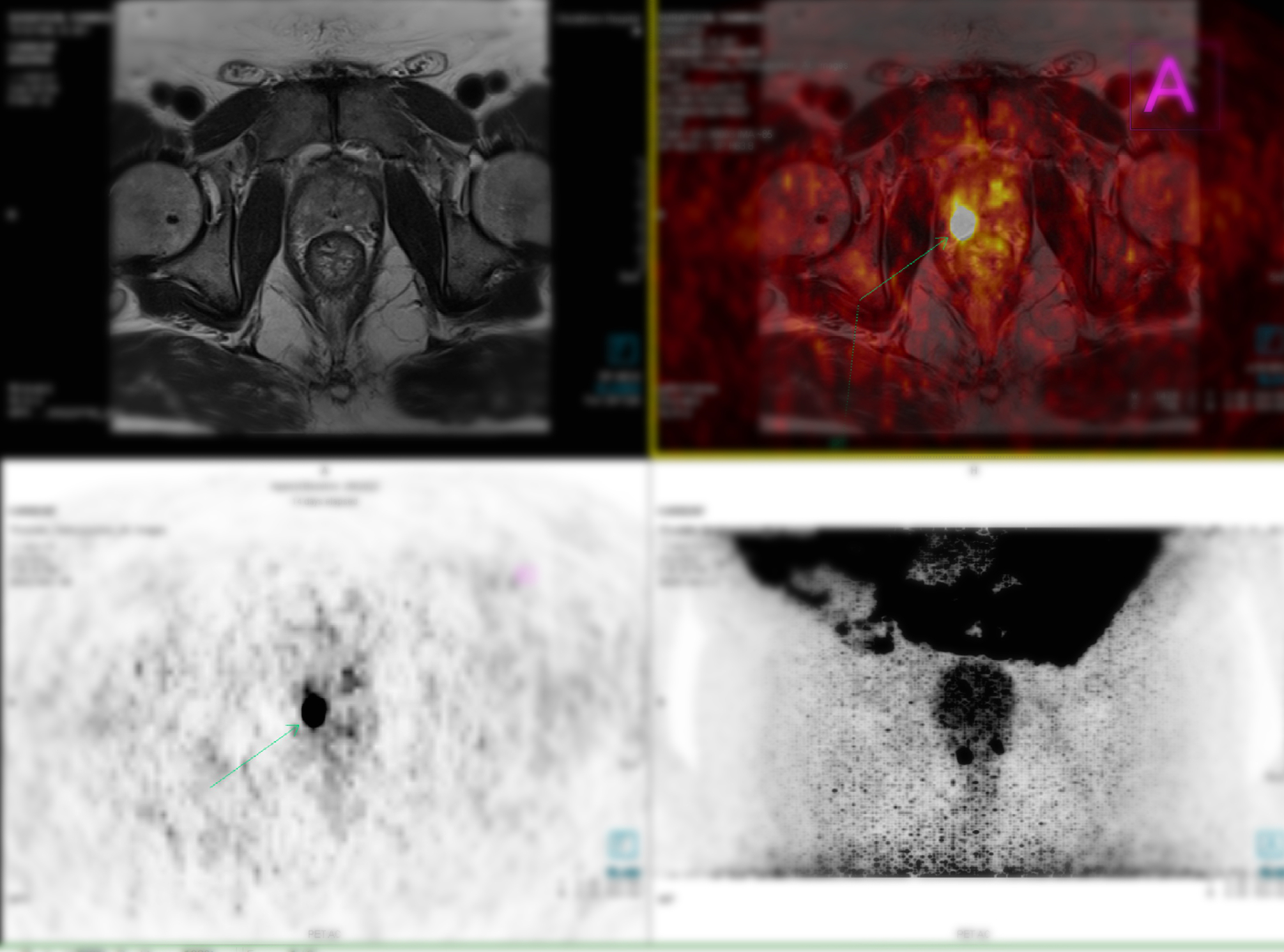
Gozetotide, also referred to in research settings as 68Ga-PSMA-11, is a radiolabelled ligand designed to bind selectively to prostate-specific membrane antigen (PSMA), a cell surface protein that is overexpressed in prostate cancer cells. It is administered intravenously and functions as a PET imaging agent, allowing clinicians to visualise the precise location of cancerous cells throughout the body. Unlike traditional imaging agents that lack specificity for prostate cancer, gozetotide provides high-contrast visualisation of tumour sites, improving the clinician’s ability to detect even small or previously occult metastases.
This agent uses the radioactive isotope gallium-68, which emits positrons detectable by PET scanners. Once injected, gozetotide circulates in the bloodstream and binds to PSMA-positive cells. The accumulation of radioactivity at these sites is then captured by a PET scan, offering a detailed map of cancer distribution.
Clinical Evidence and Comparative Efficacy
Clinical trials and retrospective studies have demonstrated that PSMA PET imaging with gozetotide significantly outperforms standard imaging in detecting prostate cancer recurrence and metastasis. One of the pivotal studies leading to its approval was conducted in patients with biochemical recurrence of prostate cancer, where rising PSA levels after treatment suggested relapse but conventional imaging failed to localise the disease.
In these trials, gozetotide PET scans identified sites of recurrence in over 75% of patients with PSA levels as low as 0.5 ng/mL. Furthermore, in patients undergoing initial staging, the agent enabled more accurate identification of lymph node involvement and distant metastases, resulting in changes in clinical management in a considerable proportion of cases.
Compared to other available PET tracers, such as choline or fluciclovine, gozetotide demonstrated superior tumour-to-background ratios, leading to higher image clarity and diagnostic confidence. Its favourable safety profile, with low incidence of adverse reactions, adds to its appeal in routine clinical use.
Regulatory Approval and International Adoption
In 2020, the United States Food and Drug Administration (FDA) granted approval for 68Ga-PSMA-11 based on compelling clinical data from academic PET centres. Since then, its adoption has expanded internationally, with multiple countries in Europe, including Germany and Italy, integrating gozetotide into clinical practice. Most recently, the UK Medicines and Healthcare products Regulatory Agency (MHRA) approved the use of gozetotide for prostate cancer imaging, marking a major step forward in British nuclear medicine services.
The approval process in the UK was accelerated by evidence from European multicentre trials and strong advocacy from oncological and nuclear medicine societies. The National Institute for Health and Care Excellence (NICE) is currently assessing its inclusion in national diagnostic guidelines, with the expectation that NHS access will become standard within a short timeframe.
Clinical Applications and Impact on Patient Care
Gozetotide is particularly useful in two major clinical scenarios: initial staging of newly diagnosed high-risk prostate cancer and localisation of recurrence in patients with rising PSA after primary therapy. Its high sensitivity and specificity enable earlier detection of metastases, allowing for timely intervention and more informed treatment planning.
For example, a patient with newly diagnosed prostate cancer and a PSA level of 20 ng/mL might be expected to undergo standard imaging with CT and bone scan. These techniques might miss micrometastatic disease, potentially leading to under-treatment. A gozetotide PET scan, however, could reveal distant spread that alters the clinical approach from curative intent to systemic therapy.
Similarly, in the context of recurrence, identifying a solitary site of relapse could allow for targeted therapies such as salvage radiotherapy, rather than systemic treatment with its associated side effects. This form of precision imaging enables a more nuanced and individualised approach to prostate cancer care, aligning with the broader movement towards personalised medicine.
Integration into NHS Services and Logistics
Adopting gozetotide PET imaging in the NHS requires strategic coordination. Gallium-68 has a relatively short half-life of 68 minutes, necessitating on-site or near-site production via a germanium/gallium generator or a cyclotron facility. While some UK hospitals already possess the necessary infrastructure, broader implementation may require investment in radiopharmacy services or collaboration with commercial radiotracer providers.
Several leading UK centres, including University College London Hospitals and The Christie in Manchester, have already conducted research using 68Ga-PSMA and are well-placed to lead clinical deployment. Additionally, training of nuclear medicine technologists, radiologists, and urologists will be key to maximising the benefits of this technology.
It is also worth noting that reimbursement structures for radiopharmaceuticals and PET imaging will need to evolve to support the wider adoption of PSMA-targeted scans. Currently, access to PET scanning is variable across the UK, and ensuring equitable access to gozetotide imaging will be a priority for healthcare planners.
The Broader Impact on Prostate Cancer Management
The approval of gozetotide not only enhances diagnostic precision but also paves the way for future theranostic approaches in oncology. Theranostics refers to the pairing of a diagnostic agent with a therapeutic analogue, often using the same molecular target. In the case of PSMA, therapeutic versions of the ligand labelled with beta or alpha-emitting isotopes (such as lutetium-177 or actinium-225) are already being trialled and used in selected settings.
This means that the same molecule used for imaging could potentially be used to deliver targeted radiotherapy to metastatic prostate cancer cells, sparing healthy tissues and reducing systemic toxicity. The imaging scan effectively acts as a ‘test dose’, demonstrating whether the cancer expresses sufficient PSMA to justify therapy.
Therefore, gozetotide’s approval has implications beyond imaging—it forms part of a broader shift towards biomarker-driven, image-guided cancer care. For patients with prostate cancer, this may mean more accurate diagnoses, fewer unnecessary treatments, and improved outcomes.
Future Developments and Research
Although the benefits of gozetotide are clear, research continues into ways to enhance its efficacy and accessibility. One focus is on developing alternative labelling isotopes such as fluorine-18, which has a longer half-life and can be produced in larger quantities. This would make PSMA PET imaging more widely available, especially in regions lacking on-site generator capability.
Another area of interest is combining PSMA PET findings with artificial intelligence to aid image interpretation and streamline reporting. Automated segmentation of tumour burden and AI-assisted risk stratification could further increase diagnostic efficiency.
In addition, researchers are investigating whether PSMA expression can predict treatment response or prognosis. If confirmed, this would enhance its utility as a biomarker and inform therapeutic decisions even more directly.
Finally, the use of PSMA PET is being expanded to other malignancies that express PSMA, such as renal cell carcinoma and certain salivary gland tumours. However, prostate cancer remains its primary and most validated application.
A Defining Moment for Prostate Cancer Imaging
The approval and adoption of gozetotide for prostate cancer diagnosis marks a defining moment in medical imaging and oncological care. Its ability to provide accurate, PSMA-targeted PET scans enables clinicians to detect, stage, and monitor prostate cancer with unprecedented clarity. As part of a broader movement towards precision medicine and theranostics, gozetotide is not merely a new diagnostic tool but a gateway to more personalised and effective cancer treatment pathways.
With increasing availability in the UK and integration into clinical practice, gozetotide offers hope for thousands of men facing prostate cancer each year. Its introduction signals a commitment to leveraging cutting-edge science for the benefit of patients, delivering care that is both more targeted and more humane. As the technology evolves and access expands, gozetotide is poised to become a cornerstone of modern prostate cancer management.
Disclaimer
The information contained in this article is provided for general informational and scientific purposes only. It is not intended as, nor should it be construed to be, medical advice, clinical guidance, or a substitute for consultation with qualified healthcare professionals. Readers must not rely on this content to make decisions regarding diagnosis, treatment, or the use of any medicinal products.
Open Medscience makes no representations or warranties, express or implied, about the completeness, accuracy, reliability, suitability, or availability of the information contained herein. While the article references published research and regulatory decisions, medical science is continually evolving, and newer data may supersede information presented at the time of publication.
The mention of specific drugs, imaging agents, institutions, or procedures does not imply endorsement or recommendation by Open Medscience. Regulatory approvals and clinical practices may vary between countries and over time. Readers are strongly advised to consult licensed medical practitioners and regulatory authorities in their jurisdiction before making any healthcare-related decisions or acting on any information contained in this publication.
To the fullest extent permitted by law, Open Medscience, its authors, and associated parties disclaim all liability for any direct, indirect, incidental, or consequential damages or losses arising from the use of, or reliance on, the information contained in this article. Use of this publication is at the reader’s own risk.
home » blog » radiopharmaceuticals »