Summary: Peptide Receptor Radionuclide Therapy (PRRT) is an advanced, targeted treatment for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). This article explores the underlying principles of PRRT, its mechanisms, clinical applications, and emerging advancements. PRRT is a form of systemic molecular therapy that targets somatostatin receptors overexpressed in GEP-NETs, delivering radiolabelled isotopes to tumour sites with remarkable precision. The treatment has shown significant efficacy in slowing tumour progression, improving symptoms, and enhancing patient quality of life. This article provides a brief overview of the therapy, its benefits, potential side effects, and future directions in research and clinical practice.
Introduction to Gastroenteropancreatic Neuroendocrine Tumours
Gastroenteropancreatic neuroendocrine tumours (GEP-NETs) are rare and heterogeneous malignancies arising from the neuroendocrine cells of the gastrointestinal tract and pancreas. These tumours exhibit varied clinical behaviours, ranging from indolent growth to aggressive progression. Common symptoms include flushing, diarrhoea, abdominal pain, and hormonal dysregulation caused by excessive secretion of bioactive substances.
Standard treatment options include surgery, medical management with somatostatin analogues, and systemic therapies such as chemotherapy. However, due to the heterogeneity of GEP-NETs and their frequent presentation in advanced stages, treatment options can be limited. This is where Peptide Receptor Radionuclide Therapy (PRRT) has emerged as a promising option.
The Basics of PRRT
PRRT is a molecular therapy designed to target tumours that overexpress somatostatin receptors, particularly the subtype 2 (SSTR2). These receptors are found on the surface of many GEP-NET cells. The therapy involves the intravenous administration of a radiolabelled somatostatin analogue, such as lutetium-177 DOTATATE or yttrium-90 DOTATOC.
The mechanism of PRRT involves:
- Targeting Receptors: The somatostatin analogue binds specifically to the somatostatin receptors on the tumour cells.
- Radiation Delivery: The attached radionuclide delivers beta-emitting radiation directly to the tumour, inducing DNA damage and cell death while sparing surrounding healthy tissues.
- Cellular Uptake: Once bound, the complex is internalised by the tumour cells, further enhancing the efficacy of radiation delivery.
Mechanisms of Action
The success of PRRT lies in its specificity and the use of radiopharmaceuticals. Key components include:
- Somatostatin Analogues: Molecules such as DOTATATE and DOTATOC are synthetic analogues with high affinity for somatostatin receptors. These serve as vehicles for radionuclides.
- Radionuclides: Lutetium-177 and yttrium-90 are the most commonly used isotopes. 177Lu is preferred for smaller tumours due to its shorter range of radiation, whereas 90Y is more effective against larger lesions.
When injected into the bloodstream, the radiopharmaceuticals home in on tumours, minimising systemic exposure. Beta particles emitted by the radionuclides cause double-stranded DNA breaks, leading to apoptosis or mitotic catastrophe.
Clinical Applications and Efficacy
Indications for PRRT
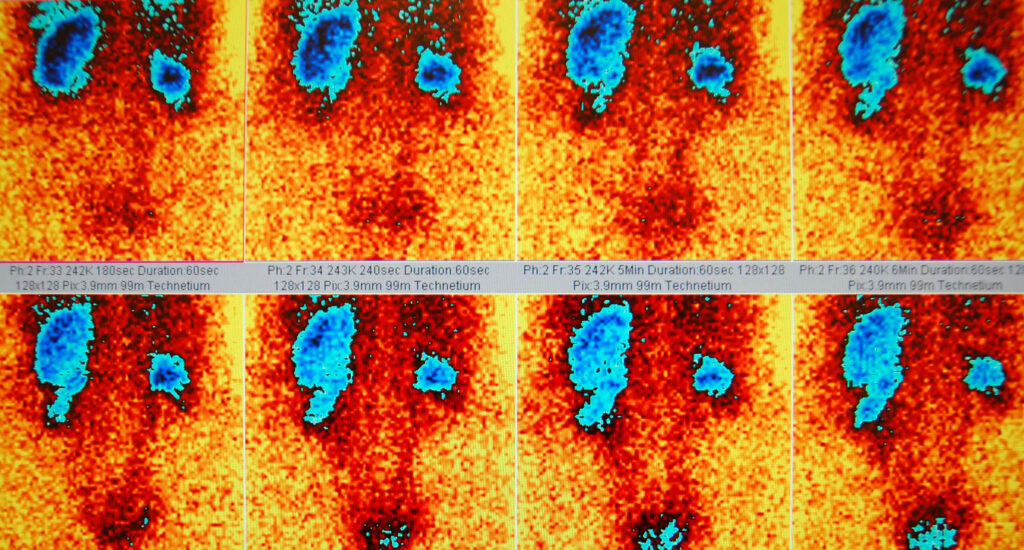
PRRT is indicated for patients with advanced or metastatic GEP-NETs that are inoperable and exhibit high somatostatin receptor expression. Patients are typically selected based on imaging studies using somatostatin receptor positron emission tomography (PET) scans.
Clinical Trials and Outcomes
Numerous studies have demonstrated the efficacy of PRRT in treating GEP-NETs. The NETTER-1 trial, a pivotal study, evaluated 177Lu-DOTATATE in patients with midgut NETs. Key findings included:
- Progression-Free Survival (PFS): PRRT significantly extended PFS compared to high-dose octreotide therapy.
- Overall Survival (OS): A trend towards improved OS was observed, although statistical significance was not reached.
- Symptom Control: PRRT alleviated symptoms such as diarrhoea and flushing in hormonally active tumours.
- Quality of Life: Patients reported marked improvements in their physical and emotional well-being.
Administration and Protocol
PRRT is typically administered in cycles, with a standard regimen involving four doses given 6–8 weeks apart. The procedure is performed in specialised centres under the supervision of a multidisciplinary team. Key steps include:
Pre-Treatment Assessment:
- Evaluation of somatostatin receptor expression using imaging.
- Assessment of kidney and bone marrow function to determine patient eligibility.
Therapy Administration:
- Radiopharmaceuticals are infused over 30–60 minutes.
- Concurrent administration of amino acid infusions helps protect the kidneys from radiation-induced damage.
Post-Treatment Monitoring:
- Regular follow-up to assess tumour response, side effects, and organ function.
Benefits of PRRT
PRRT offers several advantages over conventional therapies:
- Targeted Approach: The therapy selectively targets tumours, minimising off-target effects.
- Improved Outcomes: PRRT has demonstrated substantial benefits in terms of tumour control and symptom relief.
- Customisable Treatment: Dosimetry-guided protocols allow for personalised therapy based on individual patient needs.
Side Effects and Risks
Like any therapy, PRRT is associated with potential side effects, which are generally mild and manageable. Common adverse effects include:
- Nausea and Vomiting: Often related to the amino acid infusion.
- Bone Marrow Suppression: Leading to mild anaemia, thrombocytopenia, or leukopenia.
- Renal Toxicity: Due to the uptake of radionuclides in the kidneys.
- Long-Term Risks: A small risk of secondary malignancies has been reported, although this is rare.
Proactive management strategies, including regular monitoring and dose adjustments, mitigate these risks.
Advancements in PRRT
Alpha-Emitter PRRT
Research is exploring the use of alpha-emitting radionuclides like actinium-225, which deliver higher energy over a shorter range, potentially improving efficacy while reducing collateral damage.
Combination Therapies
Combining PRRT with other modalities, such as immune checkpoint inhibitors or chemotherapy, is under investigation to enhance tumour control.
Personalised Dosimetry
Advances in dosimetry enable precise measurement of radiation doses delivered to tumours and critical organs, allowing for personalised and optimised treatment regimens.
Next-Generation Radiopharmaceuticals
New somatostatin analogues and radionuclides are being developed to target a broader range of tumours and improve therapeutic outcomes.
Challenges and Future Directions
Despite its success, PRRT faces challenges such as:
- Limited Availability: Access to PRRT is restricted to specialised centres due to the need for advanced facilities and expertise.
- Tumour Heterogeneity: Not all GEP-NETs express sufficient somatostatin receptors for effective treatment.
- Resistance Mechanisms: Some tumours may develop resistance to PRRT, necessitating further research into overcoming this issue.
Future research aims to address these limitations and expand the applicability of PRRT. Ongoing clinical trials are investigating novel combinations, optimised protocols, and emerging technologies.
Conclusion – Peptide Receptor Therapy
Peptide Receptor Radionuclide Therapy represents a paradigm shift in the treatment of gastroenteropancreatic neuroendocrine tumours. By harnessing the power of molecular targeting, PRRT delivers precision therapy with remarkable efficacy and minimal systemic toxicity. While challenges remain, advancements in research and technology continue to expand its potential, offering hope for improved outcomes in patients with this challenging disease.
This therapy exemplifies the growing role of personalised medicine in oncology, underscoring the importance of continued investment in innovative approaches to cancer care. For patients with GEP-NETs, PRRT is not just a treatment—it is a beacon of hope for a better quality of life and longer survival.
Disclaimer
The content provided in this article, “Peptide Receptor Radionuclide Therapy (PRRT): A Breakthrough in Treating Gastroenteropancreatic Neuroendocrine Tumours”, is for informational purposes only and is intended to promote understanding of scientific and medical developments. It does not constitute medical advice, diagnosis, or treatment recommendations. Readers should not rely solely on the information presented and are strongly advised to consult a qualified healthcare professional before making any decisions about medical care or treatment options.
While every effort has been made to ensure accuracy, Open Medscience makes no representations or warranties regarding the completeness, accuracy, or reliability of the information. PRRT is a specialised therapy that may not be suitable for all patients, and its application should be considered within the context of individual clinical circumstances.
Any references to clinical trials, studies, or treatment protocols reflect current understanding at the time of writing and may be subject to change as new research emerges. Open Medscience disclaims any liability for outcomes resulting from the use or interpretation of this information.
home » blog » radiotherapeutics »