Summary: Proton therapy has attracted considerable attention in the field of cancer treatment due to its precision in targeting tumours while minimising harm to surrounding tissues. This method employs protons rather than the traditional X-rays used in conventional radiotherapy, presenting a range of potential benefits for patients. Even though it holds much promise, it also faces a variety of technical, financial, and clinical obstacles. These issues stem from the sophisticated technology required, the considerable cost of setting up and running facilities, and the ongoing need for more extensive clinical evidence. This article explores the main challenges associated with proton therapy and discusses prospective developments for addressing these hurdles.
Keywords: Proton therapy; Radiotherapy; Cancer treatment; Technological hurdles; Cost barriers; Clinical research.
Introduction to Proton Therapy
Proton therapy represents a specialised form of radiotherapy that utilises high-energy proton beams to eradicate cancer cells. Unlike conventional radiotherapy, which uses photons (X-rays), proton beams have a unique physical characteristic known as the Bragg peak. When protons travel through the body, they release a minimal amount of energy until they reach a certain depth, at which point they deposit the bulk of their energy in a concentrated burst. This feature is a key factor that makes proton therapy an attractive approach for treating certain types of cancer, as it can reduce exposure of healthy tissues and organs to radiation.
The concept of using protons for cancer treatment dates back to the mid-20th century, and the first patients received proton therapy in the late 1950s. However, it was not until the 1990s and early 2000s that interest in this therapy grew significantly. Improvements in medical imaging, accelerator design, and computer-based planning have contributed to the increase in proton treatment centres worldwide. These centres aim to broaden the range of patients who could benefit from proton therapy. Yet, although research supports the potential advantages of proton therapy, its actual role in routine clinical practice remains a subject of debate due to the hurdles detailed in the following sections.
Since proton therapy delivers radiation more selectively to the target area, it holds promise for tumours situated close to critical structures such as the spinal cord, brain stem, or key organs where sparing healthy tissue is highly beneficial. Research also suggests potential benefits for paediatric patients, as children’s organs and tissues are still in development, and lowering radiation exposure to healthy tissue is crucial for reducing long-term side effects. Hence, proton therapy is particularly appealing for brain tumours, paediatric cancers, and cancers near sensitive organs.
Technical and Operational Hurdles
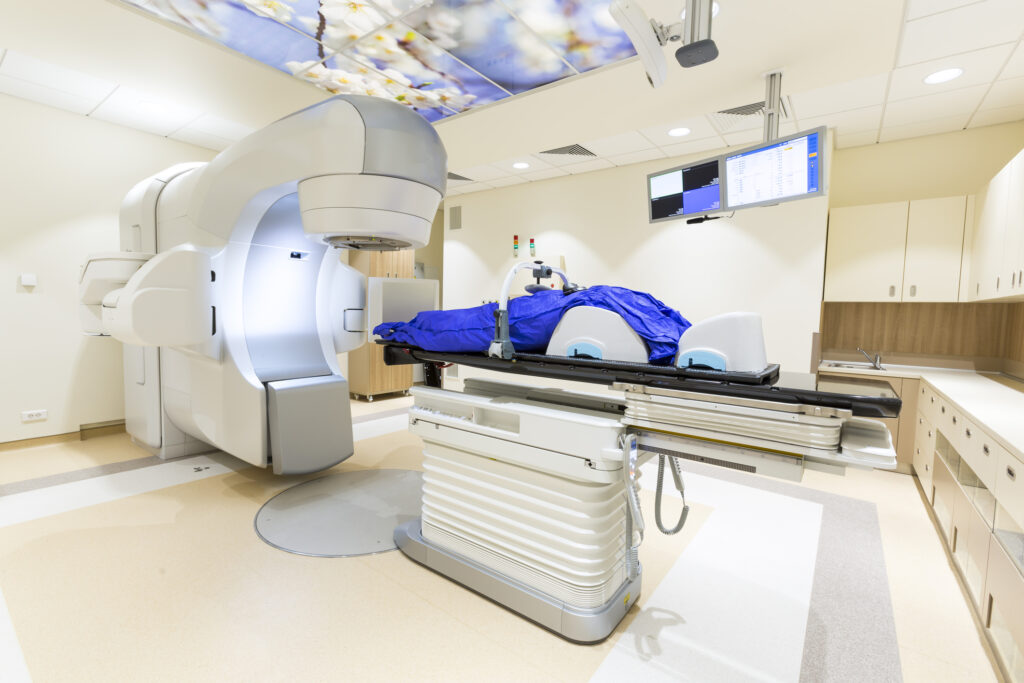
One of the fundamental challenges associated with proton therapy is the complexity of the equipment and systems used. Proton therapy machines require a particle accelerator (either a cyclotron or a synchrotron) to generate high-energy protons. These devices are not only large but also extremely expensive to build and operate. Additionally, the beam delivery mechanism is more complex than that used in standard radiotherapy because precise modulation of the proton beam’s energy is needed to match the tumour’s depth and shape.
Facilities must incorporate advanced gantries—rotating structures that direct the proton beam at the patient from various angles. These gantries are massive, often weighing several tonnes, and incorporate intricate magnetic and electronic systems. Their engineering complexity translates into higher construction and operational costs. Maintaining and calibrating such sophisticated systems demands specialised staff with advanced technical knowledge. Any small miscalculation in the energy or direction of the proton beam can affect its effectiveness or potentially damage healthy tissue. As a result, frequent quality assurance procedures and strict operational protocols are essential.
Treatment Planning and Dosimetry
Treatment planning for proton therapy is more complicated than for photon-based radiotherapy. Proton beams’ unique characteristics call for advanced imaging techniques, detailed computer simulations, and careful dose calculations. Small errors in patient positioning, organ motion, or tissue density can result in deviations in where the proton beam will deposit its energy. For instance, if a tumour moves due to breathing or digestion, the Bragg peak may not land precisely on the tumour, limiting the therapy’s effectiveness.
Moreover, materials with varying densities, such as bone, air cavities, or tissues of different compositions, can influence proton beam range. Treatment planning software must model these effects accurately. The margin for error is smaller compared to conventional radiotherapy because of the sharp dose fall-off beyond the Bragg peak. This means that advanced image-guided radiotherapy (IGRT) protocols must be used to ensure accuracy. The complexity of this planning process translates to additional time and resources, which can extend patient wait times and demand extra staff training.
Facility Infrastructure and Space Requirements
Proton therapy facilities demand substantial space. Housing a cyclotron or synchrotron, along with dedicated bunkers for radiation shielding, control rooms, and patient areas, requires more real estate than a traditional radiotherapy department. The associated shielding is also more extensive because proton beams are typically delivered at higher energies. Concrete walls can be several metres thick in certain areas, and installing them involves a massive capital investment.
Furthermore, the facility’s electrical and cooling requirements add to operational difficulties. Particle accelerators and associated systems generate substantial heat, so robust cooling systems are vital. Power supply fluctuations can disrupt sensitive equipment, and any interruption can delay patient treatments. Building or upgrading a site to accommodate such demands often entails long construction timelines and might not be viable for every healthcare institution.
Financial and Accessibility Concerns
The cost of establishing a proton therapy centre is significantly higher than that of setting up a conventional radiotherapy unit. Estimates place the price of building and equipping a single-room proton therapy facility in the tens of millions of pounds, with multi-room centres often exceeding one hundred million. This steep cost includes not only the accelerator but also gantries, beamlines, shielding, and advanced imaging systems needed for accurate treatment planning.
This initial outlay can deter many healthcare systems, especially those operating under stringent budgets. Consequently, proton therapy has predominantly been introduced in specialised research institutions or well-funded medical centres, limiting access for large segments of the population. Private funding or partnerships with industry sometimes play a role in making these centres a reality, but such partnerships can introduce questions about affordability for patients and local healthcare providers.
Operational and Maintenance Costs
Beyond the initial capital investment, running a proton therapy centre involves substantial ongoing expenses. The technological complexity, coupled with the need for highly skilled staff, pushes operational costs higher than those for photon-based treatments. Engineers, radiation physicists, dosimetrists, and specialised technicians all form part of the workforce required to keep the centre running smoothly.
Routine maintenance of the accelerator and gantries is paramount, and unplanned downtime can disrupt treatment schedules. Spare parts for highly specialised equipment are often costly and not readily available. As a result, ensuring the availability of backup components adds to the overall financial burden. Maintenance contracts can be substantial, often representing a significant fraction of the yearly operational budget.
Insurance Reimbursement and Patient Costs
Patient access to proton therapy can be affected by issues around insurance coverage and reimbursement. In some healthcare systems, including private and public payers, there is ongoing debate over whether the benefits of proton therapy justify the higher costs compared to established photon-based treatments. While it may be covered for specific indications such as paediatric tumours or rare cancers, coverage is often more limited for other conditions.
Patients might also encounter additional travel and lodging expenses if the nearest proton therapy centre is far from their home. This can be a considerable burden, especially for those who require multiple treatment sessions over several weeks. For many, the lack of local availability means choosing between standard radiotherapy near home or uprooting for an extended period to pursue proton therapy. Such dilemmas highlight the inequities in accessing cutting-edge cancer treatments.
Patient Selection and Clinical Evidence
One of the major clinical challenges for proton therapy is the limited number of large-scale, randomised clinical trials that directly compare proton therapy with photon-based radiotherapy. While there is no shortage of smaller studies, retrospective analyses, and single-institution reports, these do not always meet the highest scientific standards for establishing efficacy beyond doubt. Without robust evidence, healthcare providers and payers remain cautious about embracing proton therapy for a broad range of indications.
The rarity of randomised controlled trials is partly attributable to the prohibitive costs and logistics of running them. Research institutions willing to fund and coordinate such studies must have access to proton therapy facilities. Moreover, recruiting enough patients for meaningful statistical power can be challenging, particularly if proton therapy is restricted to only a few centres worldwide.
Disease-Specific Criteria
Proton therapy has shown promise in conditions where reducing radiation dosage to healthy tissues is especially important—like paediatric cancers. Conditions such as medulloblastoma, ependymoma, and other central nervous system tumours in children may see better long-term outcomes and fewer side effects with proton therapy. Similarly, patients with tumours close to sensitive structures (e.g., the optic nerve) may gain substantial benefits from protons’ more targeted dose distribution.
However, for many common cancers (e.g., prostate cancer, breast cancer, and lung cancer), definitive conclusions about the advantage of proton therapy over advanced photon-based techniques such as intensity-modulated radiotherapy (IMRT) are still evolving. Some research indicates lower rates of side effects, while other studies suggest only marginal differences. The absence of large-scale confirmatory data makes it difficult for oncologists to determine which patients stand to benefit the most, adding uncertainty to treatment pathways.
Ongoing Need for Real-World Data
As more proton therapy centres open and treat larger patient populations, real-world data will play a crucial role in clarifying its advantages and limitations. Registry studies, observational data, and population-based analyses can help provide valuable insights when randomised trials are not feasible. These types of data collection efforts are already underway, but it could take years for patterns to become clear regarding cancer control, survival rates, and long-term side effects.
Furthermore, data-sharing initiatives are important for expanding knowledge. When centres collaborate to pool patient outcomes, more robust conclusions can be drawn. This collaborative approach can help streamline patient selection criteria, refine treatment protocols, and guide future research, ultimately contributing to a stronger evidence base for proton therapy.
Regulatory and Training Challenges
The introduction of a proton therapy centre is subject to extensive regulatory oversight due to the high-energy radiation involved. Institutions are required to meet strict criteria for safety, shielding, and quality assurance. In many countries, obtaining approvals can take years, not only for constructing the centre but also for operating the accelerator and treating patients. Regulators must ensure that patient safety is paramount while balancing the need to enable scientific progress.
Guidelines issued by professional bodies often lag behind technological developments, as committees must gather sufficient evidence before drafting formal recommendations. This can lead to variations between centres in terms of treatment protocols, dose constraints, and patient selection. Consequently, some centres may push boundaries with novel techniques, while others adopt a more conservative approach until definitive guidelines are established.
Training and Workforce Development
An additional challenge is training the skilled workforce required to run a proton therapy facility. Radiation oncologists, medical physicists, dosimetrists, radiographers, and engineers all need specialised knowledge and hands-on experience with proton systems. Conventional radiotherapy training does not necessarily cover the nuances of proton beam therapy, including machine operation and unique treatment planning methods.
Training programmes and fellowships specifically focused on proton therapy are still developing. Practitioners often need to spend time at established centres to gain firsthand experience. Some institutions use training agreements to facilitate knowledge transfer, especially in countries where proton therapy is a relatively new venture. Building a core group of experts is fundamental for safe and effective treatment delivery, yet it is a time-consuming and resource-intensive process.
Quality Assurance and Accreditation
Because proton therapy systems are complex and expensive, maintaining high-quality standards is crucial. Quality assurance protocols must address every stage of the treatment pathway, from equipment commissioning and calibration to patient-specific treatment planning and daily verification. Any deviation in beam energy, position, or shape can have serious clinical repercussions.
Organisations such as the European Society for Radiotherapy and Oncology (ESTRO) or the American Society for Radiation Oncology (ASTRO) produce guidelines to help centres maintain best practices. Accreditation programmes also exist to certify that facilities meet the highest standards of patient safety and treatment quality. Nevertheless, achieving these standards can be a considerable burden for new centres, especially those in regions with limited existing expertise or resources. Failure to uphold these standards risks patient safety and can undermine public confidence in proton therapy.
Future Prospects and Ongoing Developments
Innovation continues at a rapid pace, and several emerging technologies could help overcome the barriers discussed earlier. One example is compact proton therapy systems, which aim to reduce the size and cost of particle accelerators. These designs use new magnet technologies or smaller synchrotrons, thereby enabling centres to be built in places that previously lacked the space or capital for large installations.
Another area of research focuses on intensity-modulated proton therapy (IMPT). This approach allows for more precise control of the proton beam, modulating its intensity pixel by pixel, which could significantly improve dose conformity. Such advancements in beam delivery and imaging techniques could enhance treatment accuracy and reduce potential side effects.
Cost Reduction Strategies
As manufacturers refine their designs and competition in the market increases, there is hope that the overall cost of proton therapy systems will decrease. Additionally, standardised, modular approaches to construction may shorten build times and lower expenses. Some hospitals are exploring models like public-private partnerships or shared facilities that serve multiple institutions. These strategies can distribute the financial risk and potentially expand patient access.
Governments and private insurers may also reconsider reimbursement structures if clinical evidence continues to demonstrate improved long-term outcomes. Reducing side effects can translate into lower healthcare costs related to managing radiation-induced complications, which could make proton therapy more appealing from an economic standpoint.
Expanding Clinical Research
A growing number of prospective and randomised clinical trials are in progress or in the planning stages. These focus on conditions such as lung cancer, breast cancer, and head and neck cancers, alongside continuing research in paediatric tumours. Over time, the data from these studies should help refine patient selection criteria and demonstrate whether proton therapy offers clear advantages over photon-based radiotherapy in different clinical scenarios.
Moreover, the increasing volume of real-world data from existing centres will shed light on long-term outcomes, side effects, and cost-effectiveness. Large registries and consortia are vital for gathering these data in a uniform manner. Ultimately, as evidence accumulates, the medical community will be better positioned to offer transparent guidance on the use of proton therapy.
Integrating Proton Therapy into Multimodal Cancer Treatment
Proton therapy is rarely used in isolation; it is typically part of a comprehensive cancer treatment strategy that may involve surgery, chemotherapy, and other targeted therapies. Exploring how proton therapy interacts with these other modalities is a key area of interest. For example, certain chemotherapy agents can act as radiosensitisers, making tumour cells more vulnerable to radiation. Determining how to combine these agents with proton therapy best could broaden the range of cancers that respond favourably.
Personalised medicine approaches are also on the horizon. Genetic profiling of tumours and advanced imaging techniques could guide more customised treatments. If specific genetic mutations make tumours more susceptible to proton therapy, identifying these markers might improve patient selection and outcomes. Combined with immunotherapy, proton therapy may elicit an enhanced immune response against cancer cells. Although such synergistic treatments are still being researched, they point to a future in which proton therapy could be integrated into a more targeted, personalised care plan.
International Collaboration and Knowledge Sharing
International collaboration is another way to accelerate progress. Shared clinical data, technology transfers, and multinational research trials can help standardise treatment protocols and clarify the role of proton therapy in modern oncology. Organisations such as the Particle Therapy Co-Operative Group (PTCOG) facilitate information exchange, conduct workshops, and guide best practices. Similarly, online platforms for data sharing and telemedicine can link professionals around the globe, enabling more informed and consistent treatment decisions.
As more countries establish their own proton therapy centres, global learning will expand. Lessons learned in the first wave of centres—regarding patient selection, cost management, and regulatory procedures—will guide newcomers to adopt more efficient and effective methods. This will potentially reduce errors, shorten learning curves, and ultimately improve patient outcomes.
Addressing Ethical and Equity Concerns
Finally, ethical considerations will become increasingly important as proton therapy evolves. Equity of access remains a pressing issue: should expensive therapies like proton therapy be reserved for conditions with the strongest supporting evidence, or should they be offered more broadly to allow for experiential learning and data collection? Balancing scientific advancement with fair access is a challenge faced by health authorities worldwide.
Moreover, communicating the potential benefits and limitations of proton therapy to patients is essential for informed decision-making. In an era where patients often research treatments online, clinicians need to provide transparent information about potential side effects, uncertainties, and alternative therapies. Only with transparent dialogue can patients feel confident in their choices and trust that they are receiving the care best suited to their clinical situation.
Conclusion
Proton therapy stands as one of the most technologically advanced approaches to cancer treatment, promising the ability to zero in on tumours while minimising harm to surrounding tissues. Its theoretical and demonstrated benefits, particularly for paediatric tumours and cancers near sensitive structures, have driven global interest in expanding access to this therapy. However, it faces a series of formidable challenges that span technical, financial, clinical, and regulatory domains.
Technical barriers include the daunting scale and complexity of proton therapy equipment, from massive accelerators to sophisticated planning systems. These challenges naturally tie into the high financial costs of construction, maintenance, and staff training, which can inhibit the spread of proton therapy facilities. Furthermore, clinical adoption is hindered by the limited number of large-scale, randomised trials, leaving certain questions unanswered about which patients truly benefit most.
Regulatory requirements are stringent, and they move at a slower pace than the rapid advances in technology. The workforce must also be trained to manage these specialised treatment systems, which adds complexity for healthcare institutions. Concurrently, there is an ongoing need for real-world data to supplement or substitute for randomised trials in cases where such studies are not feasible.
Nevertheless, the future of proton therapy looks promising. Technological innovations aim to produce smaller, more affordable machines, and competition among manufacturers could help bring costs down. Growing bodies of clinical evidence and real-world data are expected to refine patient selection criteria, making the therapy more targeted and effective. The potential for combining proton therapy with chemotherapy, immunotherapy, and genetic profiling points to an era of more personalised cancer care.
Collaboration and knowledge sharing remain integral to progress. International consortia, multi-institutional trials, and professional organisations provide frameworks for the exchange of data and best practices. As more centres open worldwide, they will benefit from the experiences of established facilities, reducing the learning curve and helping unify treatment protocols.
From an ethical standpoint, challenges remain in ensuring equitable access, particularly in healthcare systems constrained by limited resources. Policymakers, clinicians, and patient advocacy groups must weigh the high costs against the potential for reduced long-term side effects and improved quality of life. Balancing innovation with responsible stewardship of healthcare budgets is essential, as is clear communication with patients who may be considering or seeking advanced forms of cancer therapy.
In summary, proton therapy’s evolution will likely hinge on resolving technical barriers, generating stronger clinical evidence, and bringing down costs to enable broader adoption. The coming years and decades will show whether proton therapy will become a mainstream option for a wide range of cancers, or whether it will remain a specialised technique reserved for those who stand to gain the most from its unique properties. Through continuous research, technological progress, and collaborative efforts, the hope is that more cancer patients worldwide may eventually benefit from the precision and promise of proton therapy.
Q&A on Proton Therapy
1. What is proton therapy, and how does it differ from conventional radiotherapy?
Answer: Proton therapy is a specialised form of radiotherapy where protons (subatomic particles with a positive charge) are directed at a tumour. In comparison to conventional radiotherapy, which uses photons (X-rays), protons release most of their energy at a specific depth known as the Bragg peak. This precision helps target the tumour more accurately, thereby limiting radiation exposure to surrounding healthy tissues.
2. Why is proton therapy considered suitable for certain types of cancer, particularly in children?
Answer: Proton therapy’s ability to deliver radiation selectively to the tumour makes it especially helpful for tumours located near critical structures. In children, where organs and tissues are still developing, this targeted approach can reduce the likelihood of long-term side effects. Consequently, paediatric brain tumours and other cancers in close proximity to sensitive structures may see significant advantages from proton treatment.
3. What are the major technical obstacles associated with proton therapy?
Answer: One of the biggest technical hurdles is the size and complexity of the equipment used, such as cyclotrons or synchrotrons. These machines are large, costly to build, and need rigorous maintenance. Additionally, treatment planning for proton therapy is more complicated due to the necessity of accurately modelling the proton beam’s path through tissues of varying density. Regular quality assurance measures and advanced imaging techniques are needed to ensure safe and effective treatment.
4. How do the financial requirements of proton therapy centres affect their accessibility?
Answer: Building and operating a proton therapy centre can cost tens or even hundreds of millions of pounds. This high capital outlay often restricts proton therapy to specialised research institutions or well-funded medical centres. Operational and maintenance expenses, along with the requirement for highly trained personnel, further increase costs. Consequently, many patients may not have local access to proton therapy, and insurance coverage can be limited for certain cancer types.
5. What is the current state of clinical evidence supporting proton therapy?
Answer: Although multiple smaller studies indicate positive outcomes, the number of large-scale randomised clinical trials directly comparing proton therapy with standard photon-based treatments is limited. This has made certain insurers and healthcare providers hesitant to offer coverage for a wide range of indications. Nevertheless, growing real-world data, registry studies, and ongoing trials are expected to strengthen the evidence base and guide more precise patient selection criteria.
6. Why is maintaining a skilled workforce essential for proton therapy?
Answer: Proton therapy involves intricate equipment and unique treatment planning processes. Radiation oncologists, medical physicists, dosimetrists, radiographers, and engineers all require specialised training to ensure accurate patient positioning, beam calibration, and delivery. Gaining this expertise can be time-consuming and resource-intensive, but is vital for achieving consistent treatment results and upholding patient safety.
7. What are some of the regulatory and safety concerns linked to proton therapy?
Answer: Due to the high-energy beams used in proton therapy, regulatory bodies impose strict safety guidelines. Facilities must meet requirements for shielding, machine calibration, and ongoing quality checks. Obtaining the necessary approvals to build and operate a proton therapy centre can take years. Each facility must also maintain thorough safety protocols to prevent any deviation in beam accuracy, which can have serious implications for patient care.
8. Are there any technological advancements that could help reduce the cost and size of proton therapy equipment?
Answer: Researchers and manufacturers are developing compact proton therapy systems that use smaller synchrotrons or innovative magnet technology. These systems aim to reduce both the physical space required and the overall cost of building and equipping a centre. By making the technology more accessible, healthcare providers may be able to offer proton therapy to a broader range of patients in more locations.
9. How does combining proton therapy with other cancer treatments improve outcomes?
Answer: Proton therapy is typically part of a multimodal approach alongside chemotherapy, targeted therapies, and surgery. In some cases, chemotherapy agents can heighten tumour sensitivity to radiation, potentially enhancing the effectiveness of proton therapy. Ongoing research is also exploring whether immunotherapy and genetic profiling can be combined with proton treatment to maximise therapeutic benefits and reduce side effects.
10. What does the future hold for proton therapy?
Answer: The future appears promising as clinical trials expand and real-world data becomes more readily available. Technological advancements continue to refine beam delivery, reduce equipment size, and lower costs. Collaboration across international networks, improved training pathways, and clearer guidelines will further shape the role of proton therapy in modern oncology. Over time, these developments may enable broader acceptance, improved affordability, and more personalised treatment approaches for cancer patients.
Disclaimer
The content provided in this article, Proton Therapy: An Examination of Challenges and Future Directions, is intended for informational and educational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. The views expressed are those of the authors and contributors and do not necessarily reflect the official policy or position of Open Medscience or its affiliates.
Readers should not use the information in this publication to make decisions about their personal healthcare without consulting a qualified healthcare professional. While every effort has been made to ensure the accuracy and currency of the information presented, Open Medscience makes no representations or warranties of any kind, express or implied, regarding the completeness, accuracy, reliability, or suitability of the information contained herein.
Open Medscience does not endorse any specific medical institutions, technologies, treatments, or services mentioned in the article. The mention of commercial products, trade names, or organisations does not imply endorsement.
Open Medscience shall not be held liable for any direct or indirect loss or damage arising from the use or reliance on the information contained in this article.
home » blog » radiation therapy »