Radioactive Decay
Radioactive decay is a fundamental process by which unstable atomic nuclei lose energy by emitting radiation. This spontaneous transformation leads to converting one chemical element into another and is a key concept in nuclear physics and chemistry.
At its core, radioactive decay is driven by the quantum mechanical properties of atoms. The nucleus of an atom, composed of protons and neutrons, can reach a state where it is energetically favourable for it to change. This can occur through various mechanisms, such as alpha decay, beta decay, and gamma decay, each involving the emission of different particles or electromagnetic waves.
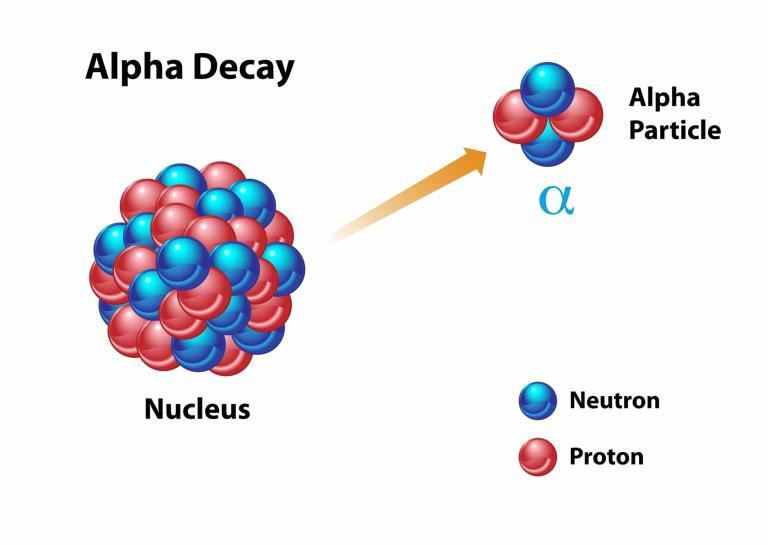
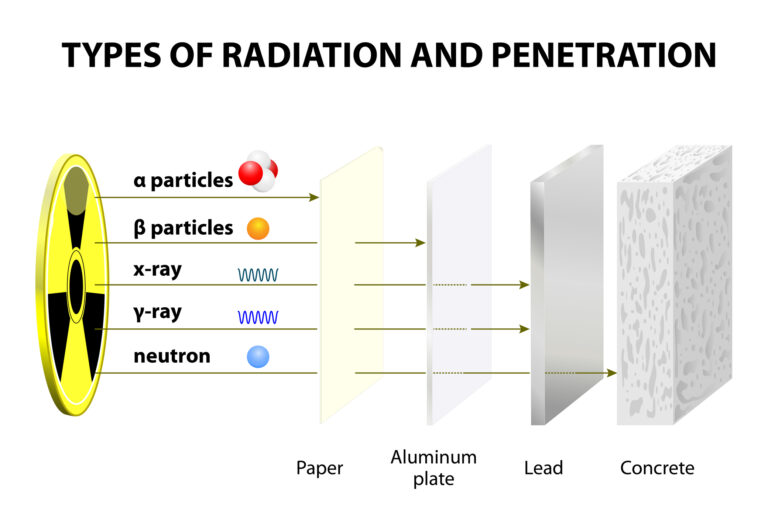
Alpha decay involves the ejection of an alpha particle, which is essentially a helium nucleus consisting of two protons and two neutrons. This type of decay typically occurs in the heaviest elements where the large nucleus seeks stability by shedding mass. An example is the decay of uranium-238 into thorium-234, a process used for dating rocks and minerals in geochronology.
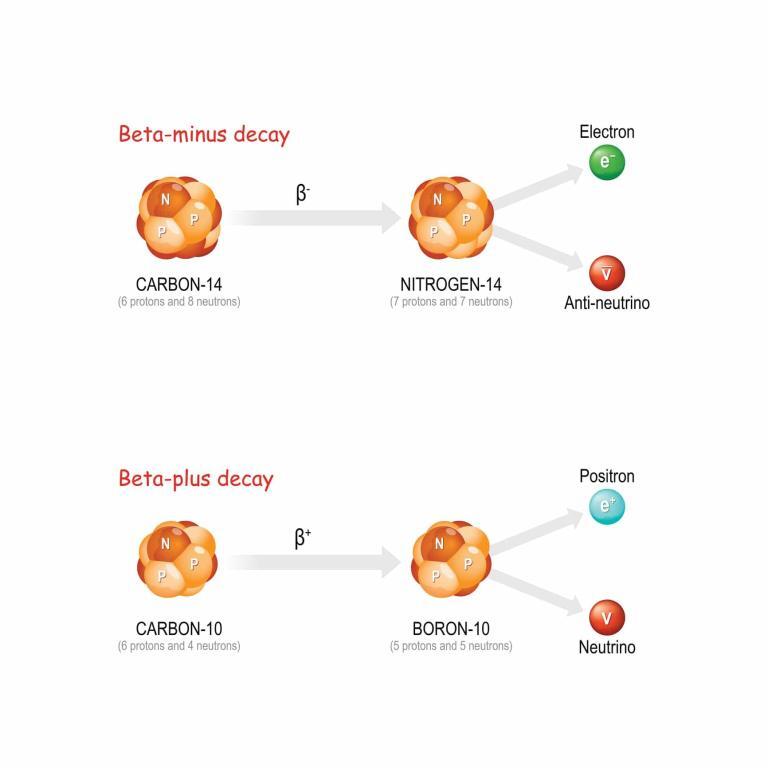
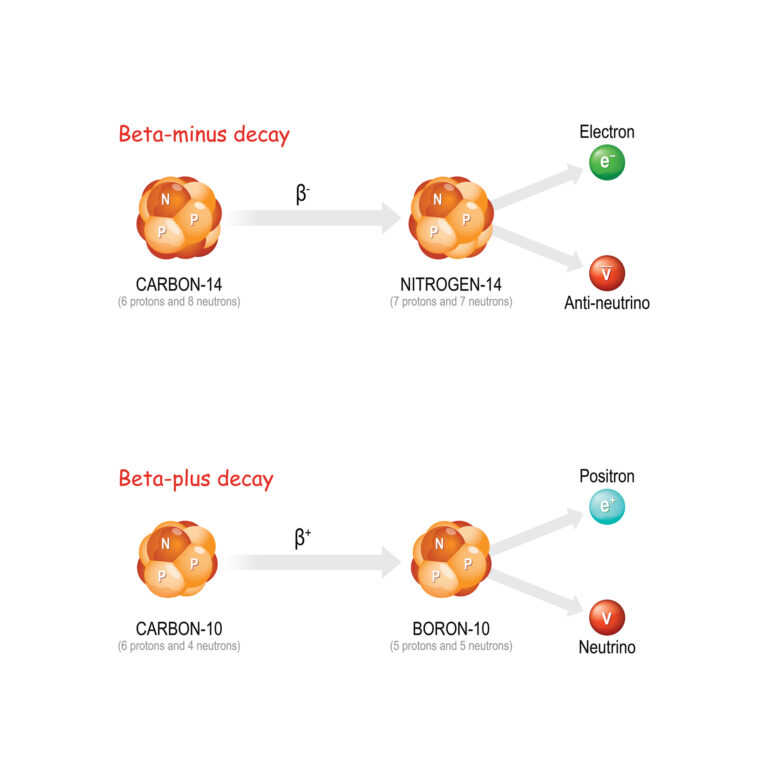
Beta decay is characterised by the transformation of a neutron into a proton or vice versa within the nucleus, accompanied by the emission of an electron (beta-minus decay) or a positron (beta-plus decay) and an antineutrino or neutrino. This decay mode is crucial for the stability of the neutron outside the nucleus and plays a significant role in the nuclear reactions that power stars.
Gamma decay, on the other hand, occurs when an excited nucleus releases excess energy in the form of gamma rays – high-energy electromagnetic radiation. This form of decay does not alter the number of protons or neutrons in the nucleus but simply moves the nucleus from a higher to a lower energy state.
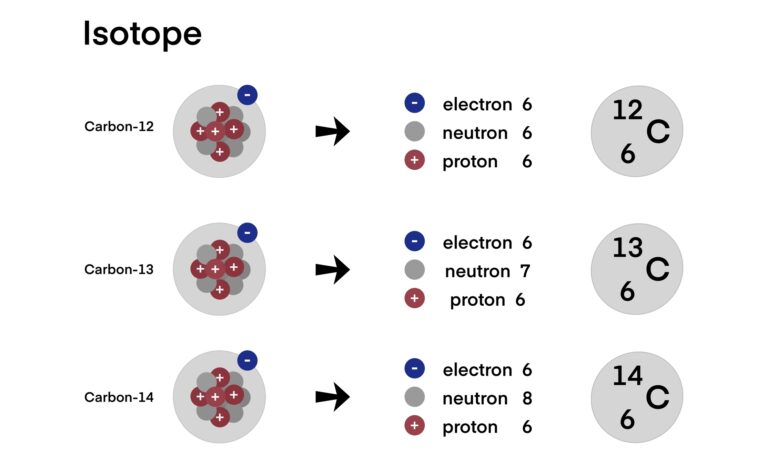
The rate at which radioactive decay occurs is measured by its half-life – the time required for half of the radioactive atoms in a sample to decay. This property is exponential in nature and varies widely among different isotopes, from fractions of a second to billions of years.
Radioactive decay is not just a subject of academic interest; it has practical applications in various fields. In medicine, it is the basis for techniques such as radioactive tracers used in diagnostic imaging and radiotherapy for treating cancers. In archaeology, carbon-14 dating, a method reliant on beta decay, is used to determine the age of organic artefacts.
Moreover, the study of radioactive decay enhances our understanding of elemental formation and transformation processes that occur in the universe. Elements heavier than iron are thought to be produced in supernovae through a series of rapid neutron capture processes known as the r-process, which is closely tied to beta decay.
In conclusion, radioactive decay is a crucial natural phenomenon with wide-reaching implications in science and technology. Enabling the transmutation of elements and providing a source of energy and diagnostic tools remains a vital area of study in understanding the physical world.
home » Radioactive Decay