Radioactivity
Radioactivity is a naturally occurring phenomenon, the result of certain atomic nuclei emitting energy in the form of particles or electromagnetic radiation. In the late 19th century, discoveries of radioactivity by Antoine Henri Becquerel marked a significant turning point in nuclear physics. It opened the door to further exploration of the atomic nucleus and its components. Radioactivity has diverse applications and consequences, from life-saving medical treatments to posing potential environmental hazards.
The radioactivity process occurs when an unstable atom’s nucleus undergoes a transformation to achieve a more stable state. This transformation can occur through three primary modes of radioactive decay: alpha, beta, and gamma.
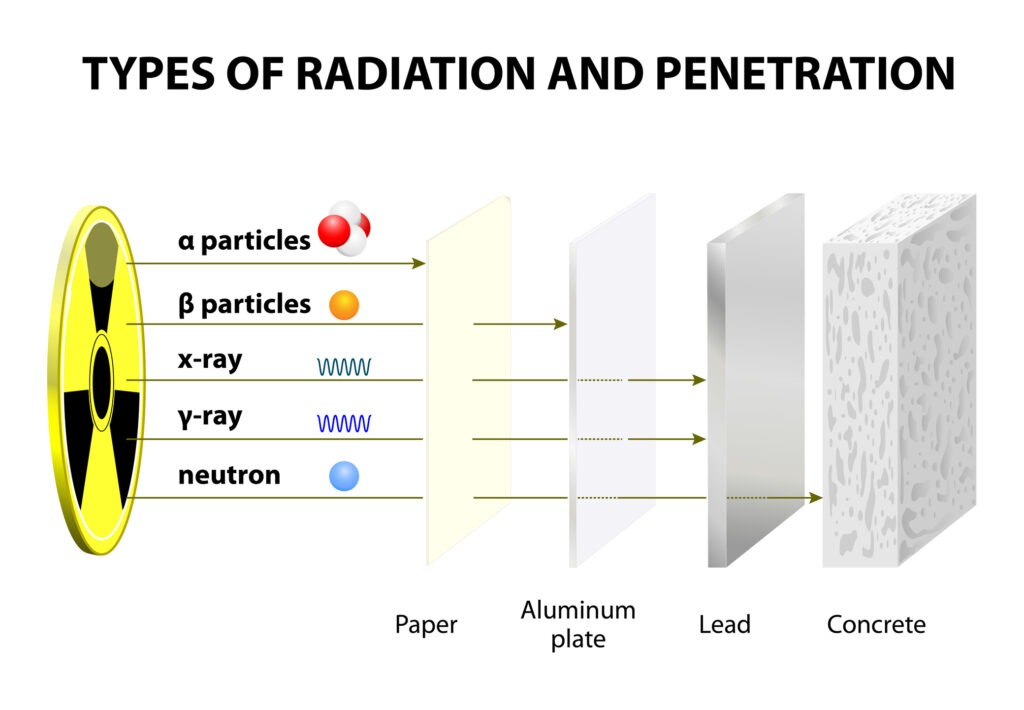
Alpha decay involves an alpha particle’s emission, consisting of two protons and two neutrons. Due to their relatively high mass and charge, alpha particles have limited penetration capabilities and are generally stopped by a sheet of paper or human skin. However, alpha-emitting materials can be extremely hazardous to living organisms if ingested or inhaled.
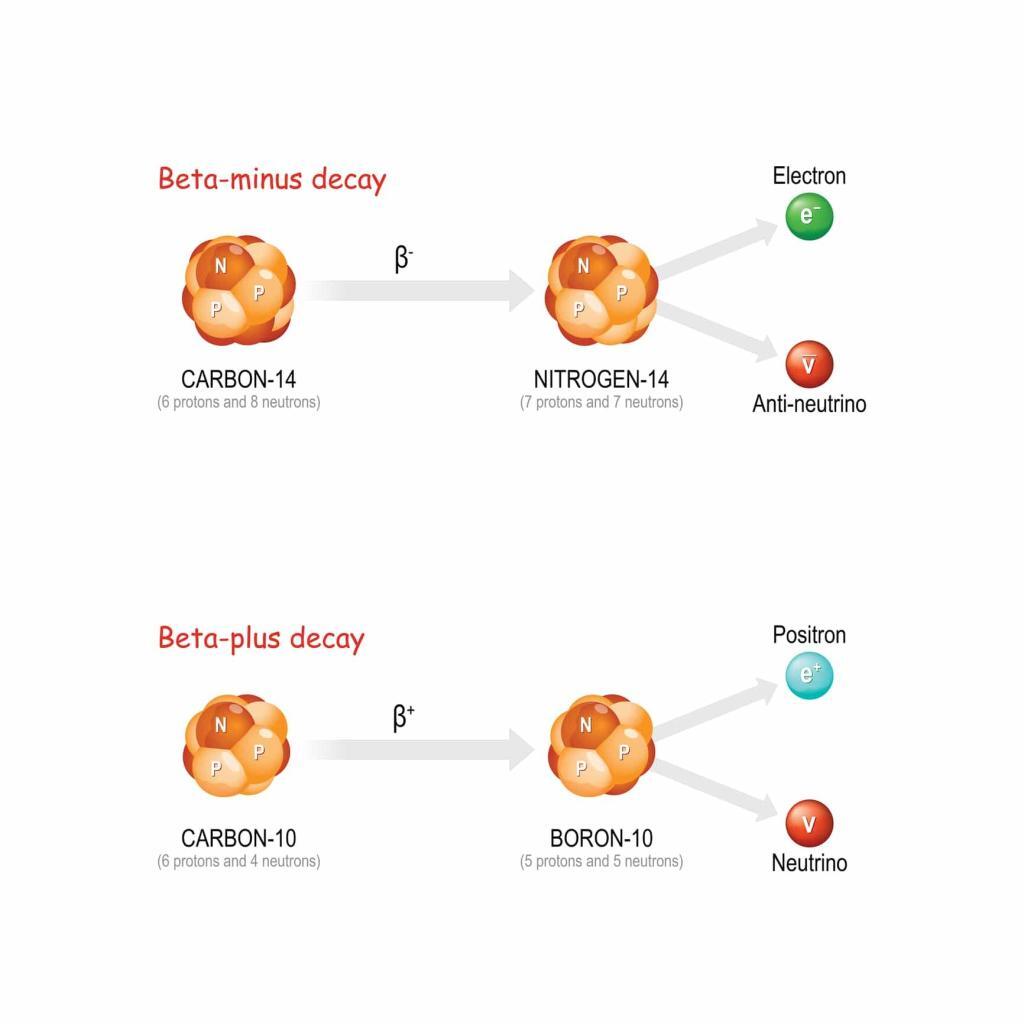
Beta decay occurs when a neutron within the nucleus is transformed into a proton or vice versa, accompanied by the emission of an electron (beta-minus decay) or positron (beta-plus decay). Beta particles have greater penetration capabilities than alpha particles but can be stopped by a few millimetres of plastic or aluminium sheet.
Gamma decay is the emission of high-energy photons known as gamma rays. These electromagnetic waves have no charge or mass and can penetrate most materials, requiring several centimetres of lead or concrete to shield them effectively. Gamma radiation is often emitted alongside alpha or beta decay as the nucleus transitions to a lower energy state.
Radioactivity has a wide array of applications, particularly in medicine and industry. For example, radioisotopes are commonly used as tracers for diagnostic purposes, as they can be easily detected when introduced into the body. Additionally, radiotherapy is an essential tool in the fight against cancer, as targeted doses of ionising radiation can damage or destroy malignant cells.
In the realm of industry, radioisotopes are employed in various processes, such as radiography, which utilises gamma radiation to inspect materials for structural integrity, and nucleonic gauges, which are used to measure the thickness or density of materials.
You are here:
home » radioactivity