- Introduction to Neuroimaging in Nuclear Medicine
- Fundamental Principles of Nuclear Medicine Neuroimaging
- Applications in Neurological Disorders
- Ethical Considerations in Neuroimaging
- Future Directions and Technological Advancements
- Clinical and Research Implications
- Challenges and Limitations
- Conclusion
Neuroimaging in nuclear medicine represents a cutting-edge convergence of technology, medicine, and neuroscience, offering unparalleled insights into the functioning of the human brain. This field utilises advanced imaging techniques, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT), to visualise and measure various physiological processes within the brain. These modalities are invaluable in diagnosing and managing neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and epilepsy. This article explores the principles of nuclear medicine neuroimaging, its applications, and the ethical considerations surrounding its use. Additionally, it addresses the future directions of the field, particularly the advancements in imaging technology and the growing role of artificial intelligence in image analysis.
Introduction to Neuroimaging in Nuclear Medicine
Neuroimaging in nuclear medicine has revolutionised the way we understand the brain’s structure and function. Unlike traditional imaging techniques that primarily focus on anatomical details, nuclear medicine neuroimaging provides functional insights by tracking the distribution of radioactive tracers in the brain. Once introduced into the body, these tracers emit radiation that can be detected by specialised imaging systems, allowing for the visualisation of brain activity in real-time.
Fundamental Principles of Nuclear Medicine Neuroimaging
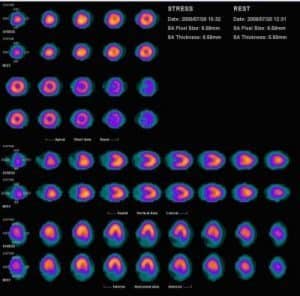
At the core of nuclear medicine neuroimaging are the techniques of PET and SPECT. Both methods rely on the detection of gamma rays emitted by radioactive tracers administered to the patient. The choice of tracer depends on the specific physiological process under investigation.
Positron Emission Tomography (PET): PET imaging involves using positron-emitting radioisotopes, such as fluorine-18, commonly used in the form of fluorodeoxyglucose (FDG). FDG-PET is particularly useful for measuring glucose metabolism in the brain, providing crucial information in diagnosing and managing conditions like Alzheimer’s disease and certain types of cancer. PET imaging is highly sensitive and provides quantitative data, making it a powerful tool in both clinical and research settings.
Single-Photon Emission Computed Tomography (SPECT): SPECT imaging, on the other hand, uses gamma-emitting radioisotopes like technetium-99m. SPECT is typically used to assess cerebral blood flow, which is an important indicator of neuronal activity. SPECT is less expensive and more widely available than PET but generally offers lower spatial resolution.
Both PET and SPECT have unique advantages and limitations, and the choice between the two often depends on the clinical question at hand.
Applications in Neurological Disorders
Nuclear medicine neuroimaging’s ability to visualise brain function makes it indispensable for diagnosing and managing various neurological disorders.
- Alzheimer’s Disease: One of the most significant applications of PET imaging is in the diagnosis of Alzheimer’s disease. FDG-PET can detect patterns of reduced glucose metabolism in the brain, which are characteristic of Alzheimer’s. Additionally, PET tracers such as amyloid-binding compounds can be used to visualise amyloid plaques, a hallmark of the disease. Early diagnosis through PET imaging allows for timely intervention, which can slow the progression of symptoms.
- Parkinson’s Disease: PET and SPECT imaging are used to assess the dopaminergic system in the brain. Tracers that bind to dopamine transporters or receptors can highlight areas of dopamine deficiency, a key feature of Parkinson’s. This information is valuable for diagnosing and monitoring the efficacy of treatments like deep brain stimulation.
- Epilepsy: In patients with epilepsy, PET and SPECT imaging can be used to identify epileptogenic foci—areas of the brain where seizures originate. This is particularly useful in cases where surgical intervention is considered. By identifying the exact location of abnormal activity, neuroimaging can guide surgeons in removing the affected tissue while preserving healthy brain function.
- Brain Tumours: PET imaging is also widely used in oncology to characterise brain tumours. FDG-PET can differentiate between benign and malignant tumours based on their metabolic activity, which is critical for treatment planning and prognosis. Additionally, PET can help monitor the response to therapy, allowing adjustments to be made if necessary.
Ethical Considerations in Neuroimaging
As with any advanced medical technology, neuroimaging in nuclear medicine raises several ethical issues that must be carefully considered.
- Radiation Exposure: One of the primary concerns is the exposure to ionising radiation. Although the doses used in PET and SPECT are generally low, repeated imaging studies can accumulate to significant levels. This is particularly relevant in vulnerable populations such as children and pregnant women. The principle of ‘as low as reasonably achievable’ (ALARA) is followed to minimise radiation exposure, but the benefits of imaging must always be weighed against the potential risks.
- Informed Consent: Given the complexity of nuclear medicine procedures, obtaining informed consent from patients is crucial. Patients must be fully informed about the nature of the procedure, the risks involved, and the potential benefits. This is especially important in research settings where the use of experimental tracers or techniques may be involved.
- Privacy and Data Security: Neuroimaging studies generate vast amounts of data, including sensitive information about an individual’s brain function. Ensuring the privacy and security of this data is paramount, particularly as the use of cloud-based storage and analysis platforms becomes more common.
- Implications of Findings: The results of neuroimaging studies can have profound implications for patients, especially in cases of degenerative diseases like Alzheimer’s, where there is currently no cure. The psychological impact of being diagnosed with such conditions must be considered, and appropriate support should be provided.
Future Directions and Technological Advancements
The field of nuclear medicine neuroimaging is rapidly evolving, with several promising developments on the horizon.
- Advancements in Tracer Development: Ongoing research is focused on developing new tracers that can target specific molecular pathways involved in neurological diseases. For instance, tau PET tracers are being developed to visualise tau protein deposits in the brain, which are associated with Alzheimer’s disease and other tauopathies. Such tracers could provide even more precise diagnostic information and help in the development of targeted therapies.
- Hybrid Imaging Techniques: The integration of PET or SPECT with other imaging modalities, such as magnetic resonance imaging (MRI), is becoming increasingly common. Hybrid imaging systems like PET-MRI combine the functional information from PET with the detailed anatomical images provided by MRI. This combination offers a more comprehensive view of the brain and can improve the accuracy of diagnoses.
- Artificial Intelligence and Machine Learning: The application of artificial intelligence (AI) in neuroimaging is a rapidly growing area. AI algorithms can be used to analyse imaging data, identify patterns that may not be immediately apparent to human observers, and even predict disease progression. Machine learning models can be trained on large datasets to improve the accuracy of diagnoses and personalise treatment plans based on individual imaging profiles.
- Quantitative Imaging Biomarkers: There is a growing interest in developing quantitative imaging biomarkers that can objectively assess disease progression and treatment response. These biomarkers could play a crucial role in clinical trials for new therapies, providing a reliable way to measure the effectiveness of interventions.
- Molecular Imaging in Neurodegenerative Diseases: Beyond Alzheimer’s disease, nuclear medicine neuroimaging is being increasingly applied to other neurodegenerative disorders, such as Huntington’s disease, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS). By visualising the underlying molecular changes associated with these conditions, neuroimaging can contribute to a better understanding of their pathophysiology and potentially lead to new therapeutic approaches.
Clinical and Research Implications
The advancements in nuclear medicine neuroimaging have significant implications for both clinical practice and research.
- Improved Diagnostic Accuracy: The ability to visualise brain function at a molecular level allows for earlier and more accurate diagnoses of neurological conditions. This is particularly important in diseases like Alzheimer’s, where early intervention can significantly improve patient outcomes.
- Personalised Medicine: Neuroimaging can provide detailed insights into an individual’s disease state, allowing for more personalised treatment approaches. For example, clinicians can tailor therapies to each patient’s unique needs by identifying specific patterns of brain activity or receptor binding.
- Guidance for Surgical Interventions: Neuroimaging plays a critical role in guiding surgical interventions in conditions such as epilepsy and brain tumours. By accurately localising areas of abnormal activity or tumour tissue, surgeons can minimise the risk of damage to healthy brain tissue, improving patient outcomes.
- Drug Development and Clinical Trials: Neuroimaging is also a valuable tool in the development of new drugs. By providing objective measures of disease progression and treatment response, imaging biomarkers can assess the efficacy of experimental therapies in clinical trials, accelerating the development of new treatments for neurological disorders.
- Longitudinal Studies and Disease Monitoring: Nuclear medicine neuroimaging is well-suited for longitudinal studies, where the same individuals are followed over time to observe disease progression or the effects of treatment. This can provide invaluable insights into the natural history of neurological conditions and the long-term efficacy of interventions.
Challenges and Limitations
While nuclear medicine neuroimaging offers numerous benefits, there are also several challenges and limitations that must be addressed.
- Cost and Accessibility: The high cost of PET and SPECT imaging and the need for specialised equipment and radiopharmaceuticals limit accessibility to these techniques. In many regions, nuclear medicine imaging is restricted to major medical centres, which can lead to disparities in healthcare access.
- Technical Limitations: Although PET and SPECT provide functional information, their spatial resolution is generally lower than that of anatomical imaging modalities like MRI. This can make it difficult to precisely localise small or deep brain structures. Efforts are ongoing to improve the resolution and sensitivity of these imaging techniques.
- Standardisation of Protocols: There is a need for greater standardisation of imaging protocols across different centres. Variability in imaging techniques and data analysis methods can lead to inconsistencies in results, making it difficult to compare findings across studies or apply them to clinical practice.
- Tracer Availability: The production and distribution of radioactive tracers require specialised facilities, and not all tracers are readily available in every region. This can limit the types of studies that can be performed, particularly in remote or resource-limited settings.
- Interpretation of Results: The interpretation of neuroimaging results requires specialised training and expertise. The complexity of the data and the potential for artefacts or false positives necessitate careful analysis by experienced professionals. The growing use of AI tools may assist in this regard, but human oversight remains essential.
Conclusion
Neuroimaging in nuclear medicine represents a powerful intersection of technology and medicine, offering profound insights into the functioning of the human brain. The techniques of PET and SPECT have transformed the diagnosis and management of neurological disorders, providing clinicians with valuable tools to visualise brain function at a molecular level. While there are challenges to be addressed, including issues of cost, accessibility, and radiation exposure, the future of neuroimaging in nuclear medicine is bright, with ongoing advancements in technology and methodology. The continued development of new tracers, hybrid imaging systems, and AI-based analysis tools promises to further enhance our understanding of the brain and improve patient care. As the field evolves, it will be crucial to balance the benefits of these powerful techniques with ethical considerations, ensuring that the use of neuroimaging in nuclear medicine continues to advance in a responsible and patient-centred manner.
You are here: home » medical imaging blog »