Radioactivity is a natural phenomenon that plays a vital role in various scientific and medical applications. To fully comprehend its effects, measurements of radioactivity must be expressed in specific units. This article explores the key units used in the field of radioactivity, including the becquerel (Bq), curie (Ci), gray (Gy), sievert (Sv), and rad, along with their historical origins, definitions, and relevance in different contexts. It also provides insight into the importance of these units in safety, medical treatments, and industrial applications.
Introduction to Radioactivity
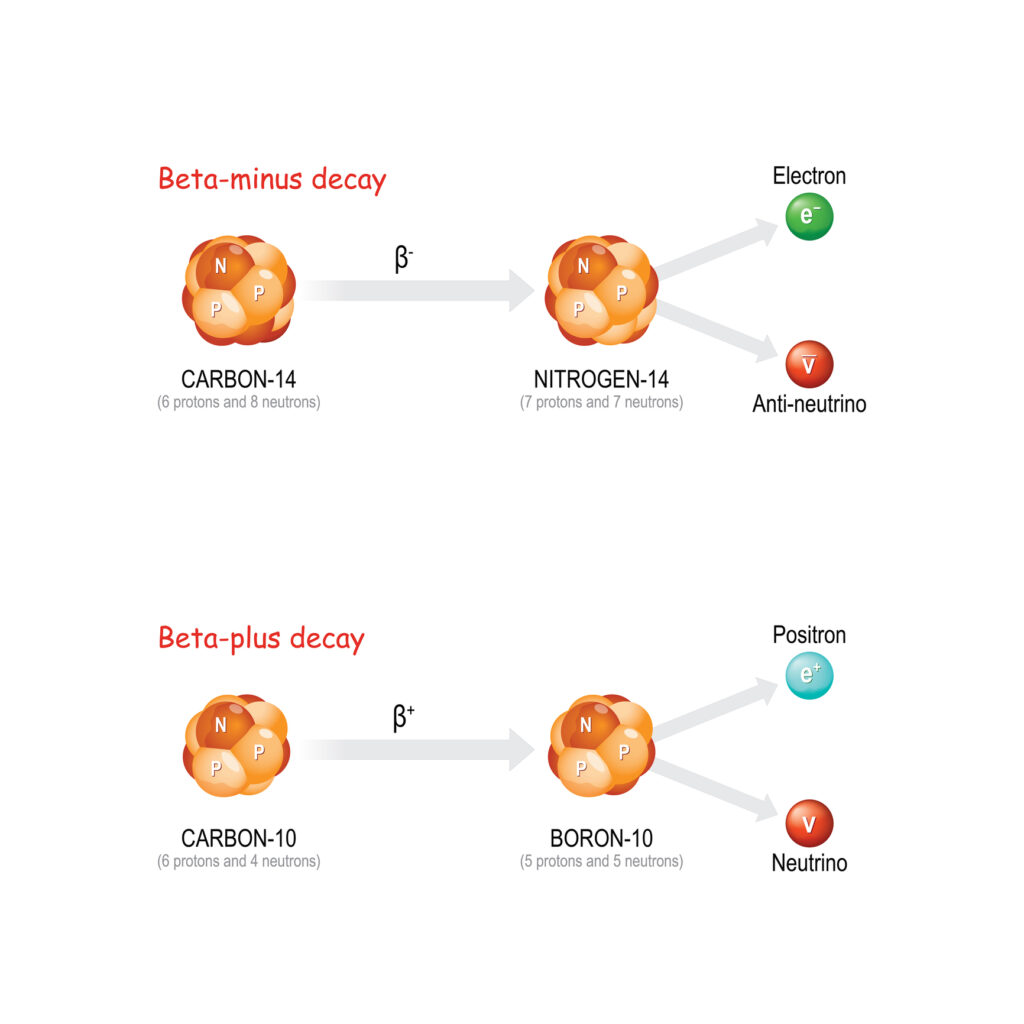
Radioactivity refers to the spontaneous emission of particles or electromagnetic waves from the unstable nuclei of certain atoms. These emissions, which are primarily in the form of alpha particles, beta particles, and gamma rays, carry energy that can interact with surrounding materials, sometimes causing significant changes or damage at the atomic level.
To measure the intensity and effects of radioactive emissions, a clear system of units is essential. Over time, scientists have developed standard units that quantify different aspects of radioactivity, including the rate of decay, the energy absorbed by materials, and the biological effects on living organisms.
The Becquerel (Bq): The SI Unit of Radioactivity
The becquerel (Bq) is the SI unit of radioactivity, named in honour of Henri Becquerel, who discovered radioactivity in 1896. One becquerel is defined as one disintegration or nuclear decay event per second. In simple terms, it quantifies the rate at which a sample of radioactive material is undergoing transformations in its atomic nuclei.
Applications
The becquerel is widely used in scientific research, medical diagnosticshttps://openmedscience.com/precision-in-treatment-the-role-of-dosimetry-in-radionuclide-therapy/, and environmental monitoring. It is particularly useful for describing the activity of low-level radioactive materials found in environmental samples or medical imaging tracers.
Practical Examples
To give a sense of scale, the natural radioactivity in a banana due to potassium-40 is about 15 Bq. In contrast, high-level radioactive waste might emit trillions of becquerels.
The Curie (Ci): The Traditional Unit
Before the adoption of the becquerel, the curie (Ci) was the most commonly used unit of radioactivity. It was named after Marie and Pierre Curie, pioneers in radioactivity research. One curie is equivalent to 3.7 × 1010 disintegrations per second, which is roughly the activity of 1 gram of radium-226, the substance the Curies studied extensively.
Continued Use in the United States
Although the becquerel is the official SI unit, the curie is still in use, especially in the United States and industries that handle large quantities of radioactive materials. For instance, in nuclear power plants or radiation therapy facilities, activity levels are often expressed in curies or millicuries (mCi).
Conversion to Becquerels
For practical purposes, conversions between curies and becquerels are necessary. The conversion is straightforward:
- 1 curie (Ci) = 3.7 × 1010 Bq. This allows for consistent communication between regions or industries using different systems of measurement.
The Gray (Gy): The Unit of Absorbed Dose
The gray (Gy) is the SI unit used to measure the absorbed dose of radiation, which is the amount of energy imparted by ionising radiation to a material. It is defined as the absorption of one joule of radiation energy per kilogram of matter.
This unit is particularly important when assessing the potential damage that radiation can cause to living tissue, as the energy deposited by radiation influences biological effects.
Applications in Medicine and Industry
The gray is commonly used in radiotherapy, where it is crucial to calculate the precise dose of radiation needed to treat cancerous tissues while minimising damage to healthy tissues. It is also important in industrial radiography and other sectors where materials are exposed to radiation.
Conversion to Other Units
Historically, the rad was used to measure absorbed dose, but the gray has largely replaced it in scientific and medical contexts:
- 1 gray (Gy) = 100 rads.
The Sievert (Sv): The Unit of Dose Equivalent
While the gray measures the physical dose of radiation, the sievert (Sv) accounts for the biological effects of different types of radiation. Different types of radiation (alpha, beta, gamma) have varying effects on living organisms, even if they deliver the same physical dose. To reflect this, the sievert incorporates a “quality factor” that adjusts for the biological impact of each type of radiation.
Definition
One sievert represents a dose that would produce the same biological effect as one gray of gamma or X-rays. This unit is crucial in assessing radiation risk and ensuring the safety of workers and the public in environments where radiation is present.
Application in Radiation Protection
The sievert is widely used in regulatory settings to ensure that radiation exposure remains within safe limits. For example, occupational exposure for radiation workers is often limited to a few millisieverts (mSv) per year. Similarly, radiation dose limits for the general public are usually set in microsieverts (µSv).
Converting Between Sieverts and Grays
In practical use, the relationship between sieverts and grays depends on the type of radiation:
- 1 sievert (Sv) = 1 gray (Gy) for gamma or X-rays. For alpha particles, the same physical dose could correspond to a much higher sievert value due to the greater biological damage they cause.
The Rad: An Older Unit of Absorbed Dose
The rad (radiation absorbed dose) was historically used to measure absorbed radiation dose before being replaced by the gray. One rad corresponds to the absorption of 100 ergs of radiation energy per gram of material.
Conversion to Modern Units
Although the rad is still found in older texts and industries, it has largely been phased out in favour of the gray. The conversion between the two units is as follows:
- 1 rad = 0.01 gray (Gy).
Current Relevance
In countries that have not fully adopted the gray, or in certain industrial applications, the rad may still be encountered, but its use is steadily declining.
The Roentgen (R): Measuring Ionisation in Air
The roentgen (R) is a unit of exposure that measures the ionisation produced by X-rays or gamma rays in the air. Specifically, it quantifies the amount of electric charge produced in a kilogram of air by ionising radiation.
Historical Significance
Named after Wilhelm Röntgen, who discovered X-rays, the roentgen was once a standard unit in radiological science. However, like the rad, it has been largely replaced by the more precise measurements offered by the gray and the sievert.
Conversion and Usage
Although rarely used today, the roentgen can still be converted to more modern units. For example:
- 1 roentgen (R) ≈ 0.009 gray (Gy) in air.
Comparing and Contrasting Units
Activity vs. Absorbed Dose
The becquerel and the curie measure the activity of a radioactive source, focusing on the number of decays occurring per second. These units do not provide information about the effects of the radiation on surrounding materials or living tissue.
In contrast, the gray and the rad measure the absorbed dose, which tells us how much energy the radiation deposits into a substance. This is more useful when considering the physical damage that radiation may cause.
Dose Equivalent and Biological Impact
The sievert and its predecessors, such as the rem, adjust for the biological effects of radiation. These units are vital in fields like healthcare and radiation protection, where the impact on living organisms is the primary concern. They incorporate the differences in how various types of radiation interact with biological tissue, which makes them critical for safety standards.
The Importance of Units in Radioactivity
Accurate measurement of radioactivity is essential for maintaining safety standards. In industries like nuclear power and medicine, exposure limits are strictly regulated to protect workers and the public. Without standard units like the sievert, gray, and becquerel, it would be impossible to maintain consistent and enforceable safety protocols.
Medical Applications
In medical treatments such as radiation therapy, precise measurements of absorbed dose (in grays) are required to effectively target cancerous cells while minimising harm to healthy tissue. In diagnostic imaging, radioactive tracers are measured in becquerels to ensure the correct activity levels are administered for PET scans and other procedures.
Industrial and Environmental Monitoring
Radioactive materials are also used in industrial applications, such as radiography and material testing. Additionally, environmental agencies monitor background radiation levels to assess potential health risks from natural or man-made sources. The becquerel and sievert are crucial units in these contexts.
Conclusion
Radioactivity is a complex phenomenon, and understanding the units used to measure it is key to managing its effects safely and effectively. From the becquerel’s measure of radioactive decay to the sievert’s focus on biological impact, each unit has a distinct role in scientific, medical, and industrial applications. As we continue to explore and harness the power of radioactivity, these units remain fundamental to ensuring precision, safety, and proper regulation.
Disclaimer
The information presented in this article is intended for general educational purposes only. It is not a substitute for professional training or certification in radiation safety, health physics, or medical physics. While efforts have been made to ensure accuracy, Open Medscience does not accept responsibility for any errors, omissions, or consequences arising from the use of the information provided. Readers should consult qualified professionals or authoritative regulatory bodies for guidance related to the handling, measurement, and effects of ionising radiation in clinical, industrial, or environmental settings.
You are here: home » diagnostic medical imaging blog »