Gallium, predicted by Mendeleev, transitions from scientific curiosity to a pivotal role in medical diagnostics.
A Brief History of Gallium
Gallium, a fascinating metal with a unique history, was first predicted by Dmitri Mendeleev in 1871. Mendeleev, renowned for creating the Periodic Table of Elements, forecasted the existence and properties of several elements. He named one of these hypothetical elements “eka-aluminium” due to its anticipated similarities to aluminium.
In 1875, the French chemist Paul-Émile Lecoq de Boisbaudran successfully isolated gallium for the first time. His discovery was remarkable because it closely matched Mendeleev’s predictions, both in terms of its chemical properties and its atomic weight. This confirmation of Mendeleev’s Periodic Table was a significant milestone in the field of chemistry.
Lecoq de Boisbaudran derived the name “gallium” from the Latin “Gallia,” meaning France, his native country. Interestingly, there’s a play of words involved as well, as “Lecoq” translates to “the rooster” in French, and the Latin word for rooster is “gallus.”
Gallium’s unique properties, particularly its low melting point of about 29.76 °C (85.57 °F), which is close to room temperature, made it a subject of curiosity and research. It melts in one’s hand, making it a popular demonstration element in science classes.
Although its early discovery, gallium didn’t find significant commercial application until the 20th century. Its major breakthrough came with the semiconductor industry’s growth. Gallium’s compounds, especially gallium arsenide (GaAs) and gallium nitride (GaN), are critical in electronics and photonics. They are used in LEDs, laser diodes, solar cells, and high-frequency electronics, including mobile phones and satellite communications.
Today, gallium continues to be a material of interest, not just for its electronic applications but also for its role in medical diagnostics, particularly in the form of gallium radiopharmaceuticals for imaging and treatment of various diseases. The history of gallium, from its theoretical prediction to its practical applications, showcases the synergy between theoretical chemistry and practical technological advancements.
Gallium Radiopharmaceuticals: Pioneering Cancer Detection through Advanced Nuclear Medicine
Gallium radiopharmaceuticals represent an important category in the field of nuclear medicine, leveraging the unique properties of gallium isotopes, primarily Gallium-67 (Ga-67) and Gallium-68 (Ga-68), for diagnostic imaging purposes. These isotopes are produced in specialised facilities: Ga-68 is typically produced using a cyclotron, while Ga-67 is generated in a nuclear reactor.
The principle behind gallium radiopharmaceuticals is their ability to bind to certain types of cells in the body, particularly cancer cells, making them useful in detecting and staging various cancers. When administered to a patient, these radiopharmaceuticals circulate in the body and accumulate in areas with high metabolic activity, such as tumour sites. This accumulation is due to the unique interaction of gallium with cellular components, particularly in rapidly dividing cells.
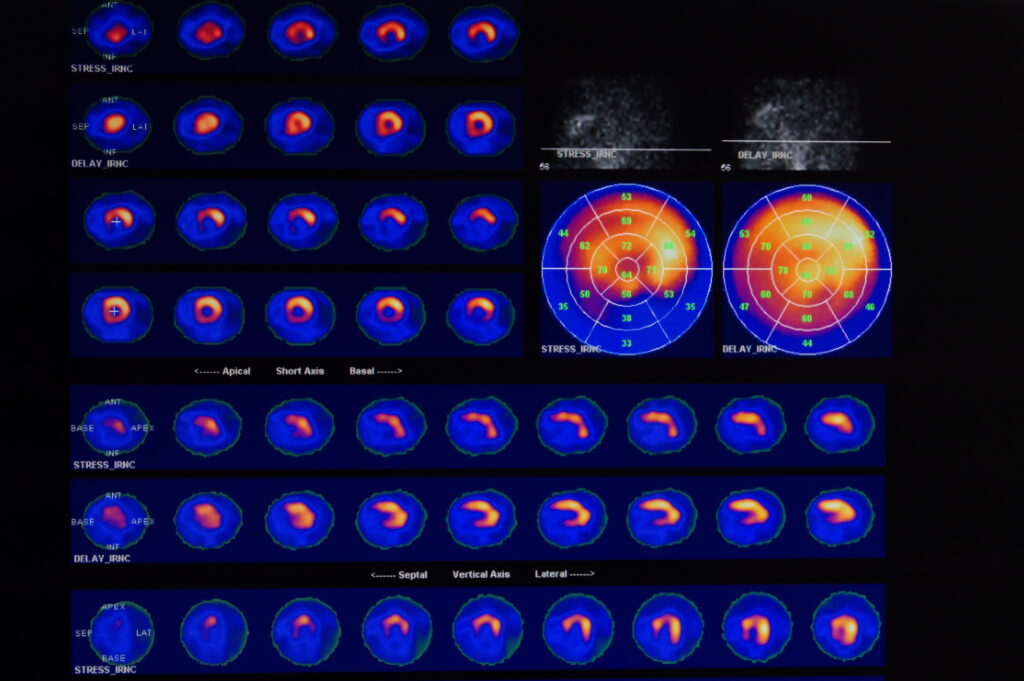
Once the gallium radiopharmaceutical is localised in the target area, its radioactive decay can be detected and imaged using specialised equipment, such as a PET scanner in the case of Ga-68 or a gamma camera for Ga-67. These imaging techniques provide detailed pictures of the body’s internal structures, highlighting areas where the radiopharmaceutical has accumulated. This information is invaluable for physicians in diagnosing, staging and monitoring the response to treatment of various cancers.
The choice between Ga-67 and Ga-68 depends on several factors, including the type of cancer, the desired resolution of the image, and the available imaging equipment. Ga-68, due to its positron emission, is commonly used in PET imaging, offering high-resolution images and a shorter half-life, which reduces radiation exposure for the patient. On the other hand, Ga-67, emitting gamma rays, is used in more traditional nuclear medicine scans.
Ga-67 and Ga-68: Harnessing Unique Isotopic Properties for Advanced Medical Imaging
Gallium isotopes, specifically Ga-67 and Ga-68, possess unique properties, making them valuable in medical imaging. Ga-67 has a relatively long half-life of about 78 hours, enabling extended imaging periods. This extended half-life, however, necessitates cautious handling due to the prolonged decay time, posing specific safety considerations. In contrast, Ga-68, with its shorter half-life of approximately 68 minutes, is well-suited for rapid diagnostic procedures. It is predominantly used in PET imaging due to its quick decay, which allows for high-resolution imaging while minimising the patient’s exposure to radiation. These distinct properties cater to different diagnostic needs in nuclear medicine.
Unveiling Disease: How Gallium Radiopharmaceuticals Mimic Iron to Target Pathological Tissues
The mechanism governing the uptake of gallium radiopharmaceuticals in tissues is intricate and not fully elucidated. Central to this process is the ability of gallium ions to mimic iron. Gallium binds to transferrin, a protein responsible for iron transport in the bloodstream.
This mimicry facilitates the accumulation of gallium in regions with heightened iron metabolism, a characteristic often found in various pathological conditions. Infections, inflammation, and tumours, for instance, exhibit increased iron metabolism due to their rapid cell division and growth, making them prime targets for gallium accumulation.
As gallium attaches to transferrin, it is transported to and concentrates in these areas, allowing for their precise identification and assessment through imaging techniques. This unique behaviour of gallium, leveraging the body’s natural iron transport mechanisms, underscores its effectiveness in detecting and localising areas of disease activity, particularly in oncological and infectious disease imaging.
Gallium Radiopharmaceuticals: Revolutionising Clinical Diagnostics in Oncology and Beyond
Gallium radiopharmaceuticals have carved a significant niche in clinical diagnostics, particularly in imaging tumours, infections, and inflammatory conditions. Their use is especially pronounced in imaging lymphomas and specific types of carcinomas, where their ability to accumulate in rapidly dividing cells provides critical diagnostic information.
In the case of lymphomas, gallium radiopharmaceuticals have shown substantial efficacy. These agents accumulate in lymphoid tissues, enabling the detailed imaging of both Hodgkin’s and non-Hodgkin’s lymphomas. This capability is vital for staging the disease, assessing treatment response, and monitoring for recurrence. Similarly, in certain carcinomas, such as lung, breast, and melanoma, gallium radiopharmaceuticals assist in detecting primary tumours and metastatic lesions, offering valuable insights for treatment planning.
Beyond oncology, gallium radiopharmaceuticals are instrumental in identifying sites of infection and inflammation. This is particularly beneficial in patients with immune deficiencies, where traditional diagnostic methods may be less effective. Gallium imaging can precisely localise infections like osteomyelitis, pulmonary infections, and chronic inflammatory conditions. The radiopharmaceuticals’ propensity to accumulate in areas of active inflammation or infection enables clinicians to distinguish between infection and tumour, a distinction that is often challenging with conventional imaging modalities.
Moreover, the use of Ga-68 has grown with the advent of PET imaging. Ga-68 PET imaging offers higher resolution and shorter scanning times compared to traditional nuclear medicine techniques, enhancing the detection and characterisation of lesions, particularly in complex clinical scenarios.
Ga-68 Revolution in PET Imaging: Enhancing Oncological Diagnostics with Superior Resolution and Sensitivity
The advent of Ga-68 in PET imaging has revolutionised the field, significantly elevating the resolution and sensitivity of gallium scans. Ga-68, with its favourable physical and chemical properties, has enabled the development of highly effective radiopharmaceuticals for PET imaging, particularly in oncology.
One of the notable advancements is the use of Ga-68 labelled compounds such as Ga-68 DOTATATE, a radiotracer specifically designed for neuroendocrine tumour (NET) imaging. NETs are a group of heterogeneous tumours that often present diagnostic challenges due to their small size and variable location. Ga-68 DOTATATE binds to somatostatin receptors, which are frequently overexpressed in NETs, allowing for highly specific and sensitive detection of these tumours.
The superior performance of Ga-68 DOTATATE in PET imaging lies in its ability to detect even small lesions that conventional imaging methods may miss. This high sensitivity and specificity level significantly improves tumour localisation, staging, and restaging accuracy, enhancing patient management. Furthermore, the short half-life of Ga-68 facilitates rapid imaging and reduces radiation exposure to the patient, aligning with the principles of radiation safety and patient comfort.
Unlocking Diagnostic Precision: The Unique Advantages of Gallium Radiopharmaceuticals in Medical Imaging
Gallium radiopharmaceuticals stand out in diagnostic imaging due to their unique advantages over other imaging agents, particularly their efficacy in detecting certain tumours and inflammatory conditions. These advantages stem from the distinctive properties of gallium and its interaction with biological systems.
One of the primary advantages is their exceptional affinity for certain types of tumours, especially lymphomas and neuroendocrine tumours. Gallium compounds can bind to specific receptors or molecules overexpressed in these tumours, allowing for a more precise and sensitive detection than conventional imaging modalities. This specificity is particularly crucial in early diagnosis, accurate staging, and monitoring of the response to therapy, directly impacting patient management and outcomes.
Additionally, gallium radiopharmaceuticals excel in identifying sites of infection and inflammation. Their uptake in areas of increased metabolic activity, a common feature of both infectious and inflammatory processes, makes them extremely useful in diagnosing and localising hidden or indolent infections, especially in immunocompromised patients. This ability is significant where other imaging techniques might fail or provide non-specific results.
Moreover, the introduction of Ga-68 in PET imaging has enhanced the resolution and sensitivity of gallium scans, offering more precise and more detailed images. This is particularly beneficial in detecting small lesions and in areas with complex anatomy where other imaging agents might provide limited information.
Challenges and Limitations in the Clinical Use of Gallium Radiopharmaceuticals in Diagnostic Imaging
Despite the significant advantages of gallium radiopharmaceuticals in diagnostic imaging, there are certain limitations and challenges associated with their use. One notable limitation is the background uptake of gallium in normal tissues. This physiological uptake, particularly in organs like the liver, spleen, and bones, can potentially obscure the interpretation of scans. Differentiating between pathological uptake and normal physiological distribution becomes challenging, and this can lead to either false positive or false negative results, particularly in areas adjacent to these organs.
Another challenge lies in the production and availability of Ga-68. Unlike other radionuclides, Ga-68 requires a cyclotron for its production, a significant infrastructure investment that is not universally available. This limitation restricts the accessibility of Ga-68-based radiopharmaceuticals to only those medical centres with cyclotron facilities. Additionally, the process of labelling compounds with Ga-68, such as Ga-68 DOTATATE for neuroendocrine tumour imaging, necessitates a specialised synthesis module. This module is crucial for the radiolabeling process but also adds to the complexity and cost of producing Ga-68 radiopharmaceuticals.
Furthermore, the short half-life of Ga-68, while beneficial in reducing radiation exposure to the patient, also imposes logistical challenges regarding timing and coordination of radiopharmaceutical synthesis, patient scheduling, and imaging.
Advancing Horizons: The Promising Future of Gallium Radiopharmaceuticals in Diagnostic Imaging
The future of gallium radiopharmaceuticals is promising, with ongoing research focused on enhancing their diagnostic capabilities. Key areas of development include creating new gallium-based agents with improved targeting abilities and reduced background uptake. Such advancements would significantly enhance the specificity and clarity of gallium scans, thereby improving diagnostic accuracy.
Innovations in radiopharmaceutical chemistry are pivotal in this regard. Researchers are exploring novel ligands and molecular structures that can bind more selectively to tumour cells or sites of infection and inflammation, minimising non-specific uptake in normal tissues. This specificity would be particularly beneficial in complex cases where current agents may produce ambiguous results.
Additionally, advancements in PET technology, including better detectors and image processing algorithms, are likely to broaden the clinical applications of gallium. Enhanced imaging capabilities could provide clearer, more detailed images, aiding in early detection and precise localisation of diseases.
These future developments in both the chemistry of gallium agents and imaging technology hold the potential to significantly expand the use of gallium in medical diagnostics, offering more accurate, efficient, and versatile imaging solutions. This progress would enhance patient care and pave the way for new applications in personalised medicine and targeted therapy.
Ensuring Safety and Compliance: The Responsible Use of Gallium Radiopharmaceuticals in Medical Imaging
Gallium radiopharmaceuticals are generally considered safe for clinical use, exhibiting a low incidence of adverse reactions. The safety profile of these agents is a significant advantage, making them well-suited for a wide range of patients. However, like all radioactive materials, they require meticulous handling and strict adherence to safety and regulatory guidelines. This ensures the safety of both patients and healthcare staff. Regulatory bodies provide clear protocols for using, storing, and disposing of radiopharmaceuticals, which must be rigorously followed.
These guidelines are designed to minimise radiation exposure to patients, staff, and the environment. Regular training and updated certifications for handling radioactive materials are also essential in maintaining a safe and compliant medical imaging environment. The overall safety of gallium radiopharmaceuticals, combined with proper handling and regulatory adherence, underscores their viability and effectiveness as diagnostic tools in modern medicine.
Conclusion
conGallium radiopharmaceuticals stand as a crucial element in the array of diagnostic imaging tools, offering unique properties and continuously evolving applications that significantly contribute to the field of nuclear medicine. Their ability to accurately diagnose and manage various diseases, particularly in oncology and infection imaging, underscores their importance. This overview has encapsulated the essence of gallium radiopharmaceuticals, shedding light on their properties, clinical applications, limitations, and the promising horizon of their future developments. As research and technology advance, these agents are poised to take on an increasingly pivotal role in the area of precision diagnostics and personalised medicine. The potential for more targeted and effective imaging agents, coupled with advancements in imaging technology, suggests that gallium radiopharmaceuticals will continue to enhance our capabilities in disease detection, staging, and monitoring, ultimately contributing to improved patient outcomes and the broader field of medical imaging.
Gallium Radiopharmaceuticals and Applications
| Radiopharmaceutical | Gallium Isotope Used | Primary Applications | Notable Features |
| Gallium Citrate Ga-67 | Gallium-67 (Ga-67) | Neuroendocrine tumours, GIST, and other receptor-positive tumors | – Long half-life (78 hours) – Good for extended imaging sessions – Used in SPECT imaging |
| Gallium DOTATATE Ga-68 | Gallium-68 (Ga-68) | Neuroendocrine tumour imaging | – Short half-life (68 minutes) – High resolution in PET scans – Superior in detecting small lesions |
| Gallium DOTANOC Ga-68 | Gallium-68 (Ga-68) | Neuroendocrine and other types of tumours | – Used for PET/CT imaging – High affinity for somatostatin receptors |
| Gallium DOTATOC Ga-68 | Gallium-68 (Ga-68) | Neuroendocrine tumours, GIST, and other receptor-positive tumours | – High sensitivity and specificity – Used in PET/CT imaging |
| Gallium MZ3 Ga-68 | Gallium-68 (Ga-68) | Prostate cancer imaging | Neuroendocrine tumours, GIST, and other receptor-positive tumours |
Disclaimer
The content provided in this article, “Gallium Radiopharmaceuticals in Medical Imaging”, is intended for informational and educational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Open MedScience does not provide medical services or engage in the practice of medicine.
The scientific information presented herein reflects current knowledge as of the publication date and may not encompass the latest developments or regulatory changes. While efforts have been made to ensure the accuracy of the content, Open MedScience makes no representations or warranties, express or implied, regarding the completeness, accuracy, or reliability of the information.
Gallium radiopharmaceuticals must only be handled and administered by qualified professionals in accordance with national and international safety regulations and under the appropriate medical and legal supervision. The use of radioactive substances involves specific risks and regulatory requirements that vary by jurisdiction. All clinical decisions should be made by licensed healthcare providers based on a comprehensive assessment of individual patient circumstances and in line with applicable standards of care.
Open MedScience disclaims any liability for any loss, injury, or damage arising from the use or misuse of information contained in this article. Readers are strongly encouraged to consult appropriate specialists and regulatory authorities before implementing any procedures or clinical decisions involving gallium radiopharmaceuticals or related nuclear medicine technologies.
home » blog » radiopharmaceuticals »