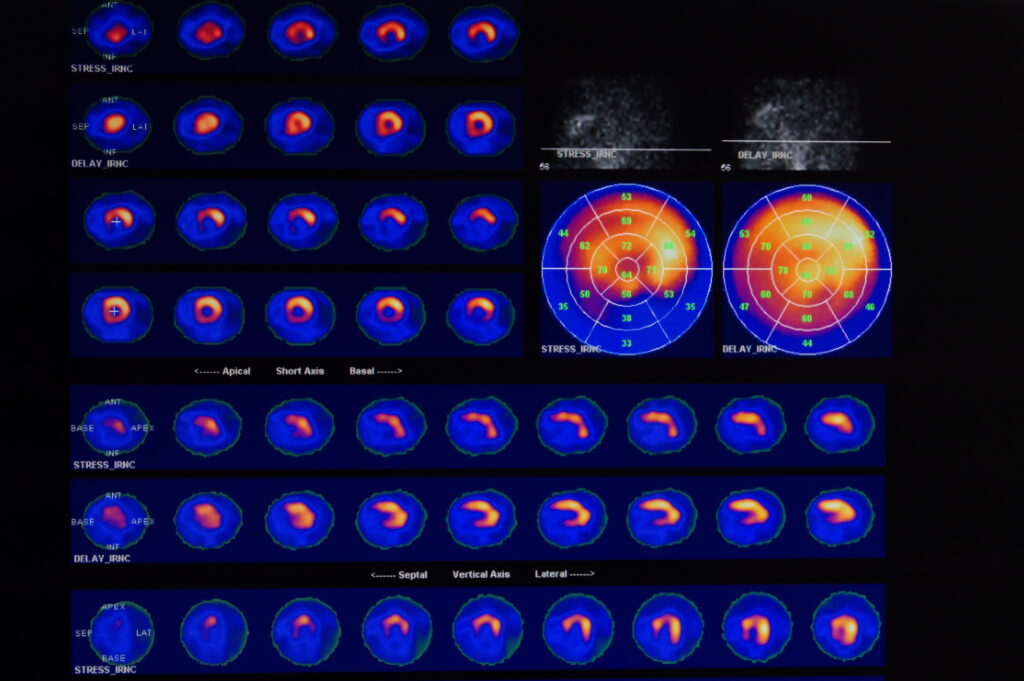
Summary: Unconjugated radionuclides are critical in nuclear medicine and radiopharmaceutical applications, offering unique capabilities for both diagnostic imaging and therapeutic interventions. Unlike conjugated counterparts, which are attached to biological carriers for targeted delivery, unconjugated radionuclides operate independently, providing distinct advantages in certain clinical contexts. This article examines the types, production methods, clinical applications, and safety considerations surrounding unconjugated radionuclides, aiming to elucidate their role in advancing medical imaging and targeted cancer therapies.
Introduction to Radionuclides in Medicine
Radionuclides play a central role in nuclear medicine, a field dedicated to using radioactive materials for both diagnosis and treatment. The utility of radionuclides spans diagnostic imaging, allowing non-invasive visualisation of internal structures and functions, and therapeutic applications, where radiation can target and destroy diseased tissue, notably cancer cells.
Radionuclides are typically categorised as either conjugated or unconjugated. Conjugated radionuclides are bound to carriers like antibodies, peptides, or small molecules to ensure specific delivery to target cells or tissues. Conversely, unconjugated radionuclides lack such targeting agents and, therefore, operate based on their inherent radiophysical properties and biodistribution characteristics.
Types of Unconjugated Radionuclides
Unconjugated radionuclides are chosen based on their decay characteristics, half-life, and energy emissions. Common radionuclides include:
- Technetium-99m: A gamma-emitting radionuclide widely used in diagnostic imaging due to its ideal half-life (six hours) and suitable energy emissions for gamma cameras.
- Iodine-131: Known for both diagnostic and therapeutic purposes, iodine-131 emits beta and gamma radiation, making it useful in treating thyroid conditions and imaging thyroid structures.
- Strontium-89 and Samarium-153: These beta-emitting radionuclides are utilised for palliative treatment of bone pain in metastatic cancers.
- Yttrium-90: Emitting beta particles, yttrium-90 is used in treating specific cancers, such as liver cancer, and in certain cases for radiosynovectomy, a procedure targeting synovial inflammation in joints.
Each of these radionuclides is selected based on the biological and physical characteristics that best match the intended clinical application, with some having wider therapeutic or diagnostic roles depending on emission type and tissue penetration depth.
Production and Preparation of Unconjugated Radionuclides
The production of radionuclides is a sophisticated process primarily conducted in nuclear reactors or cyclotrons, where stable atoms are bombarded with particles to produce radioactive isotopes. The two main production methods include:
- Nuclear Reactors: Used to produce neutron-rich radionuclides, reactors facilitate neutron activation, transforming stable isotopes into radioactive ones. Examples include iodine-131 and molybdenum-99 (the precursor for technetium-99m production).
- Cyclotrons: Producing proton-rich radionuclides, cyclotrons are more commonly used for positron-emitting isotopes used in PET imaging. The particle acceleration process allows protons to collide with stable atoms, creating radionuclides such as fluorine-18.
The methods and equipment required for radionuclide production are chosen based on the type of emission desired, the half-life of the radionuclide, and logistical factors related to its clinical application.
Clinical Applications of Unconjugated Radionuclides
Diagnostic Imaging
Unconjugated radionuclides play an invaluable role in diagnostic imaging, enabling clinicians to assess organ function, detect abnormalities, and monitor disease progression. Some examples include:
- Bone Scans: Technetium-99m, one of the most used radionuclides in bone scans, allows clinicians to identify areas of abnormal bone metabolism, useful in diagnosing fractures, infections, and metastatic bone disease.
- Thyroid Scans: Iodine-131 and iodine-123 provide specific thyroid imaging, as the thyroid gland selectively absorbs iodine. These radionuclides are utilised to assess thyroid nodules, hyperthyroidism, and thyroid cancers.
- Renal Imaging: For renal function assessment, technetium-99m-based agents are administered to evaluate glomerular filtration rate and renal blood flow. These imaging techniques help detect conditions such as obstructions, renal artery stenosis, and renal insufficiency.
These diagnostic applications rely on the precise biodistribution properties of radionuclides, which, though unconjugated, are naturally taken up by specific organs or tissues.
Therapeutic Applications
Unconjugated radionuclides are equally important in therapeutic nuclear medicine, especially in treating cancers and inflammatory conditions. Examples of therapeutic applications include:
- Thyroid Cancer Treatment: Iodine-131, with its high-energy beta emissions, is used to ablate thyroid tissue and treat thyroid cancer. The thyroid’s affinity for iodine ensures the effective delivery of radiation directly to the targeted cells.
- Bone Pain Palliation: For patients with metastatic bone pain, particularly in prostate and breast cancers, beta-emitting radionuclides like strontium-89 and samarium-153 are used. These radionuclides localise in areas of increased bone turnover, providing targeted radiation to alleviate pain.
- Liver Cancer Therapy: Yttrium-90 is used in selective internal radiation therapy (SIRT) for liver cancer. Administered directly into the hepatic artery, yttrium-90 particles become lodged within tumours, providing high-dose radiation while sparing surrounding healthy tissue.
The therapeutic use of unconjugated radionuclides is highly effective for localised treatment, especially in cases where the radiation can be selectively absorbed by specific tissues or pathological structures.
Advantages of Using Unconjugated Radionuclides
Unconjugated radionuclides offer several unique advantages in clinical practice:
- Simpler Production and Cost-Effectiveness: Without the need for complex labelling or conjugation to biological carriers, unconjugated radionuclides are generally simpler and less costly to produce.
- Immediate Biodistribution: Unconjugated radionuclides, especially those with specific biodistribution tendencies (e.g., iodine uptake in the thyroid), naturally target certain tissues without requiring additional targeting molecules.
- Flexibility in Imaging and Therapy: Certain radionuclides, such as iodine-131, serve dual purposes in both diagnostic and therapeutic applications, simplifying patient management.
- Reduced Risk of Immunogenicity: The lack of conjugation means there is no carrier protein or antibody that could potentially elicit an immune response in the patient.
These factors make unconjugated radionuclides valuable for clinical applications requiring swift radiolabel incorporation and minimal processing.
Safety and Regulatory Considerations
The use of radionuclides in medicine is heavily regulated to ensure safety for patients, healthcare workers, and the environment. Key safety and regulatory concerns include:
- Radiation Protection Protocols: Healthcare professionals handling unconjugated radionuclides must follow stringent radiation protection guidelines to limit exposure. Shielding, monitoring, and safe handling practices are mandated to protect against both external and internal contamination.
- Waste Disposal: Disposal of radioactive waste from unused or decayed radionuclides follows strict protocols to avoid environmental contamination and safeguard public health.
- Patient Safety: Administered doses are carefully calculated to balance therapeutic efficacy with safety, aiming to minimise exposure to healthy tissues while delivering effective doses to target areas.
- Transportation and Storage: Given the short half-lives of many radionuclides, they must be stored and transported under controlled conditions to prevent degradation and ensure safe, timely administration.
These regulatory frameworks are overseen by agencies such as the International Atomic Energy Agency (IAEA) and national regulatory bodies, ensuring that all steps in the use of radionuclides adhere to high safety standards.
Challenges and Future Directions
The application of unconjugated radionuclides presents certain challenges, which are areas of active research and technological development:
- Limited Targeting Precision: Unlike conjugated radionuclides, unconjugated radionuclides lack the precision targeting provided by biological carriers. This can lead to lower selectivity and potential off-target effects, particularly in therapeutic applications.
- Short Half-Lives: Radionuclides with short half-lives, while advantageous for patient safety and disposal, pose logistical challenges in transport, storage, and timely use, necessitating efficient radiopharmaceutical supply chains.
- Development of Novel Radionuclides: Researchers continue to explore new radionuclides with desirable decay properties and biodistribution patterns to expand the range of applications for unconjugated radionuclides.
- Improved Production Techniques: Advances in cyclotron and reactor technology, as well as automation in radionuclide synthesis, aim to optimise the production process, reduce costs, and improve availability.
The future of unconjugated radionuclides in nuclear medicine lies in addressing these challenges while expanding their clinical utility in both diagnostics and therapeutics.
Conclusion
Unconjugated radionuclides serve a vital role in nuclear medicine, offering versatile applications across diagnostic imaging and targeted therapies. Their inherent radiophysical properties enable unique clinical applications without the need for biological carriers, making them a valuable option in cases where immediate biodistribution or cost-effective production is paramount. However, challenges related to targeting specificity, regulatory requirements, and logistical management continue to shape their use and development.
The field of unconjugated radionuclides is advancing as researchers explore new isotopes, production methods, and applications, paving the way for innovations that could enhance both diagnostic accuracy and therapeutic efficacy in nuclear medicine. With continued research, unconjugated radionuclides are likely to remain a cornerstone of radiopharmaceutical science, benefiting a diverse range of patients through both established and emerging clinical applications.
Disclaimer
The content provided in this article is for informational and educational purposes only and does not constitute medical, scientific, or regulatory advice. While every effort has been made to ensure accuracy, Open Medscience makes no warranties or representations regarding the completeness, reliability, or applicability of the information presented. The use of unconjugated radionuclides in medical settings should always be undertaken in accordance with applicable clinical guidelines, local regulations, and under the supervision of qualified healthcare professionals. Readers are encouraged to consult with licensed experts before making decisions related to the use or handling of radionuclides. Open Medscience does not accept responsibility for any outcomes resulting from the use of the information contained within this article.
home » blog » radiopharmaceuticals »