Summary: Radioisotopes are atoms with unstable nuclei that release radiation as they transform into more stable forms. These naturally occurring or artificially produced isotopes play a critical role across a wide range of scientific and industrial fields, including medicine, energy, agriculture, archaeology, and environmental studies. This article explores the fundamental nature of radioisotopes, the science behind their behaviour, methods of production, their diverse applications, and considerations surrounding safety and regulation. With increasing reliance on nuclear technologies and a growing emphasis on medical diagnostics and treatments, understanding radioisotopes has never been more relevant.
Keywords: Radioisotopes, Radiation, Nuclear Medicine, Radioactive Decay, Isotopes, Applications
Introduction to Radioisotopes
Atoms form the basis of all matter, and each atom consists of a nucleus made up of protons and neutrons, surrounded by electrons. An element is defined by the number of protons in its nucleus, but atoms of the same element can have different numbers of neutrons. These variations are known as isotopes. While many isotopes are stable, some are not. Those that are unstable and undergo spontaneous nuclear transformations to become more stable are called radioisotopes.
This transformation process involves the release of energy in the form of radiation, a characteristic that makes radioisotopes both fascinating and highly useful. Radiation emitted can take the form of alpha particles, beta particles, or gamma rays, each with distinct properties and levels of penetration. The process by which an unstable isotope becomes stable is referred to as radioactive decay.
The Science Behind Radioactive Decay
Radioactive decay is a random but measurable process. Each radioisotope has a unique half-life — the time it takes for half of a given amount of the isotope to decay. This concept is crucial to understanding the behaviour and applications of radioisotopes. Some decay in fractions of a second, while others remain active for thousands or even millions of years.
During decay, the atomic structure changes. For example, an unstable nucleus may emit a beta particle (either an electron or a positron), thereby changing a neutron into a proton or vice versa. In doing so, the element itself transforms — a process known as transmutation. This ability to change from one element to another underlies many nuclear processes, including those involved in nuclear reactors and diagnostic imaging technologies.
Natural and Artificial Production of Radioisotopes
Some radioisotopes occur naturally. Potassium-40, carbon-14, and uranium-238 are examples of naturally occurring isotopes that have existed since the Earth’s formation. Others are produced artificially in nuclear reactors or particle accelerators. In these settings, stable atoms are bombarded with neutrons or protons, resulting in changes to their nuclei and the creation of radioactive versions of otherwise stable elements.
Nuclear reactors are the primary source of many medical and industrial radioisotopes. For instance, molybdenum-99, which decays to form technetium-99m — widely used in diagnostic imaging — is typically produced in reactors. Cyclotrons, a type of particle accelerator, are often used to produce short-lived isotopes for immediate use in hospitals.
Applications in Medicine
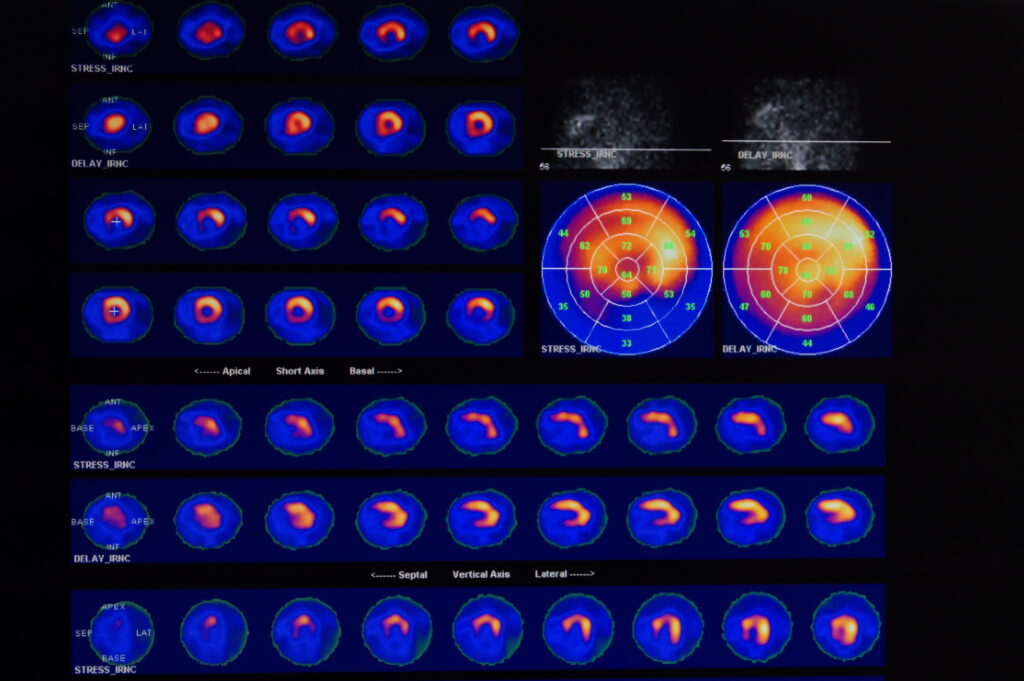
One of the most important and well-known uses of radioisotopes is in the field of medicine. In diagnostic imaging, radioisotopes help doctors visualise internal organs and detect abnormalities. For instance, technetium-99m is used in nuclear medicine scans to evaluate organ function, such as the heart, lungs, and kidneys. These scans provide vital information without the need for invasive procedures.
In oncology, radioisotopes are utilised for both diagnostic and therapeutic purposes. Positron emission tomography (PET) scans use fluorine-18 to detect cancerous growths and monitor treatment responses. Meanwhile, therapeutic radioisotopes, such as iodine-131, target specific tissues — including the thyroid gland — to treat hyperthyroidism or thyroid cancer by delivering controlled doses of radiation that destroy malignant cells.
Radiotherapy, another crucial treatment method, employs isotopes like cobalt-60 or caesium-137 to emit high-energy gamma rays that shrink or eliminate tumours. The precision of modern techniques has greatly improved, minimising damage to healthy tissues.
Industrial and Agricultural Uses
Beyond medicine, radioisotopes are widely used in industry and agriculture. In industrial settings, they serve as tracers to detect leaks in pipelines or wear and corrosion in machinery. Radiography using gamma rays from isotopes such as iridium-192 or cobalt-60 enables non-destructive inspection of metal parts and welds, which is crucial for safety in the construction, aviation, and automotive industries.
In agriculture, radioisotopes help improve crop yields and food security. They are used to induce beneficial mutations in plants, track nutrient uptake, and monitor pest control strategies. Radiation from isotopes also helps sterilise food and medical equipment, extending shelf life and ensuring safety without chemical additives.
Radioisotope tracing techniques have also transformed environmental science. They allow scientists to study water movement, soil erosion, and pollutant dispersion with remarkable accuracy.
Role in Archaeology and Geology
Radioisotopes also provide essential tools for dating historical and geological materials. Carbon-14 dating is a widely recognised method that enables archaeologists to estimate the age of organic remains up to approximately 50,000 years old. The decay of carbon-14, present in all living organisms, continues after death, enabling age calculations based on residual amounts.
For older materials, other isotopes such as potassium-40 and uranium-238 are used in techniques like potassium-argon dating and uranium-lead dating. These methods have been instrumental in understanding Earth’s history, the timing of volcanic events, and the development of early human civilisations.
Safety, Regulation and Public Perception
Although radioisotopes offer many benefits, their use requires strict safety protocols due to the health risks associated with radiation exposure. Prolonged or intense exposure can damage tissues and increase the risk of cancer. As such, workers in nuclear medicine, power plants, and research laboratories follow detailed safety procedures, including shielding, maintaining a safe distance, and managing exposure time.
Regulatory bodies such as the UK’s Office for Nuclear Regulation (ONR) and international organisations like the International Atomic Energy Agency (IAEA) oversee the use, transport, and disposal of radioactive materials. These agencies ensure that radioisotopes are handled safely and responsibly, with a focus on protecting both people and the environment.
Associations with nuclear accidents and weapons often shape public perception of radioisotopes. However, the controlled use of radioisotopes in medical and scientific applications is far removed from such contexts. Better public education about the safe and beneficial use of these products could help alleviate unfounded fears.
Environmental Considerations and Waste Management
One of the ongoing challenges in the use of radioisotopes is managing the waste produced, especially from medical and industrial applications. Radioactive waste must be carefully segregated, stored, and disposed of to prevent environmental contamination. The half-life of the isotopes in the waste determines the duration and method of storage — shorter-lived isotopes may decay to safe levels within days or weeks, while longer-lived ones require secure containment over many years.
Recycling and reprocessing technologies are being developed to reduce waste and recover usable materials. However, the infrastructure required for such efforts is complex and often expensive. As the use of radioisotopes expands, so too must the investment in environmentally sustainable methods for handling them.
Future Prospects and Innovations
As technology advances, new radioisotopes are being developed for specialised purposes. In targeted alpha therapy (TAT), isotopes such as actinium-225 are showing promise in treating cancers that are difficult to reach with minimal damage to surrounding tissues. Research is also focused on improving the delivery mechanisms for radiopharmaceuticals to enhance accuracy and reduce side effects.
In nuclear energy, understanding isotopic behaviour contributes to the development of next-generation reactors, such as thorium-based or fusion reactors, which promise safer and more sustainable energy production. Innovations in imaging and data analysis are making radioisotope-based diagnostics more accurate and accessible, particularly in low-resource settings.
Continued collaboration among scientists, regulators, and clinicians is essential to harness the full potential of radioisotopes while ensuring their safe and ethical use.
Conclusion
Radioisotopes are a cornerstone of many scientific and practical advances. From saving lives through medical diagnostics and treatments to supporting global food production, powering space missions, and uncovering the secrets of the past, their contributions are vast and far-reaching.
Understanding the nature, use, and safety of radioisotopes is crucial not only for professionals in scientific fields but also for an informed public. As the world faces new challenges in healthcare, energy, and environmental management, radioisotopes will undoubtedly continue to play a vital role in shaping innovative solutions. Their power, when understood and applied wisely, presents opportunities that impact nearly every aspect of modern life.
Disclaimer
The content of this article is intended for informational and educational purposes only. Open Medscience does not provide medical, scientific, engineering, or safety advice. While efforts have been made to ensure the accuracy and relevance of the information presented, readers should consult appropriate professionals or regulatory authorities before acting on any details related to the use, handling, or disposal of radioactive materials.
Use of radioisotopes is subject to local and international regulations, and improper handling can pose serious health and environmental risks. The article does not replace guidance from qualified practitioners, and no responsibility is accepted for any consequences arising from reliance on the content. Always follow established safety procedures and obtain necessary authorisations when working with radioactive substances.
The views expressed are those of the author(s) and do not necessarily reflect the position of Open Medscience or any associated institutions.
home » blog » radiopharmaceuticals »