Summary: This article provides an overview of beta particle emitting radionuclides that are used for cancer treatment and related disorders in therapeutic nuclear medicine. It explores their mechanisms of action, important considerations in administering them, and the significance of tissue targeting for improved results. It also addresses the production processes for suitable radiopharmaceuticals, with an emphasis on patient safety measures. The discussion highlights the selection criteria behind these beta radiation sources, ensuring that clinicians can make well-informed choices for optimal care.
Keywords: beta radiation; therapeutic nuclear medicine; radionuclides; radiopharmaceuticals; tissue targeting; patient safety.
Introduction Beta Particle Emitting Radionuclides
Beta particle emitting radionuclides play a key role in cancer therapy, particularly within therapeutic nuclear medicine. The ability of these radionuclides to deliver beta radiation directly to malignant cells enables precise intervention and can improve treatment outcomes. This approach typically involves administering specialised radiopharmaceuticals that accumulate within tumour tissues, subsequently emitting their radioactive energy to damage or destroy diseased cells. A crucial principle underlying this technique is tissue targeting, which maximises the therapeutic index by sparing healthy structures from high radiation doses.
Radioactive sources used in therapeutic nuclear medicine must satisfy several essential criteria. Their half-life should be appropriate, allowing enough time for the radiopharmaceuticals to be administered, transported, and deposited within the tumour while limiting exposure to healthy tissues. Another significant consideration is the energy level of beta radiation, as it influences how deeply the emissions penetrate tissues and the potential for undesired side effects. Ongoing research efforts strive to refine these physical characteristics, paving the way for increasingly effective and selective radionuclides. Throughout this process, preserving patient safety remains a top priority, from the earliest dosage estimations through to follow-up monitoring.
Beyond these physical characteristics, beta particle emitting radionuclides must exhibit favourable biodistribution patterns. This is generally accomplished by coupling them with biomolecules (such as peptides or antibodies) that are drawn to tumour-specific markers, providing enhanced tissue targeting. By improving the localisation of radioactive emissions, therapeutic nuclear medicine can reduce the burden of collateral damage on healthy tissues and improve patient outcomes.
Background on Beta Particle Emitting Radionuclides
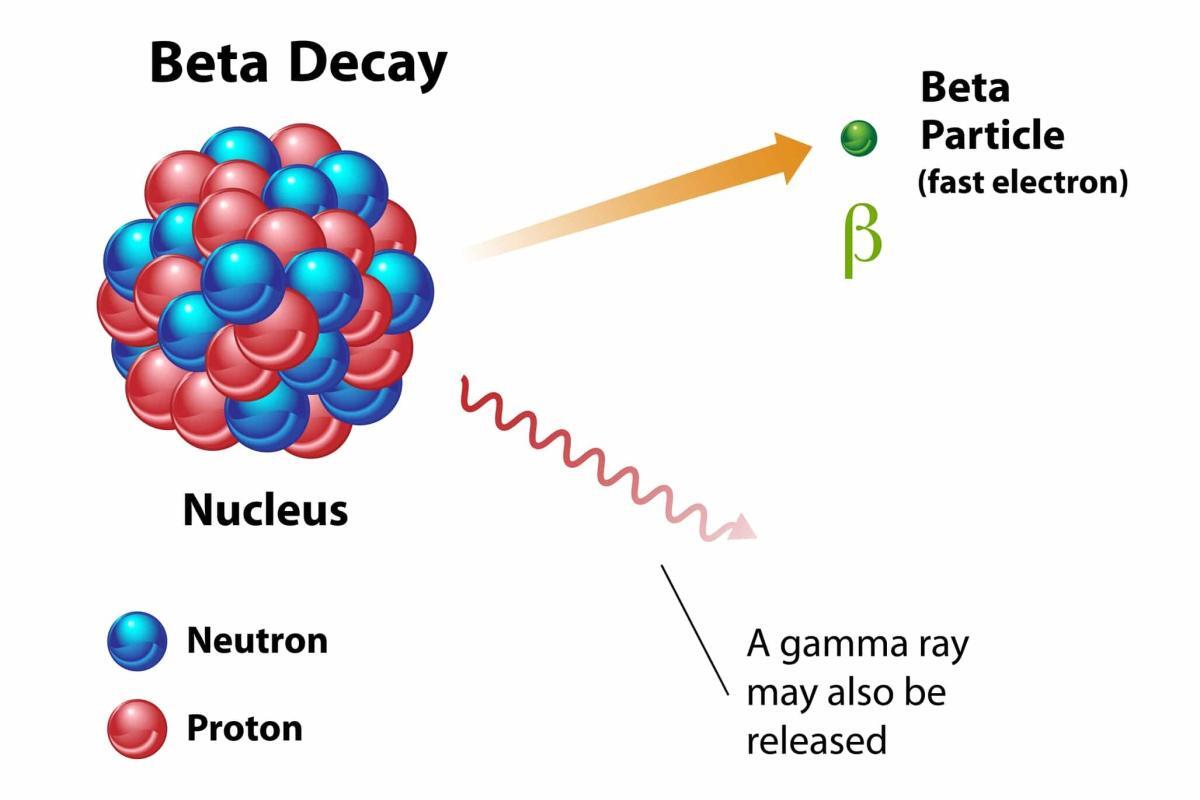
The phrase “beta particle emitting radionuclides” refers to radioactive isotopes that release electrons (beta particles) when they decay. These electrons can deposit substantial energy in local tissues, causing irreversible harm to cells and, potentially, cell death. In therapeutic nuclear medicine, this destructive capability is harnessed against malignant cells, helping to shrink tumours and, in certain settings, offer relief for patients with advanced or otherwise intractable diseases.
Prominent examples of beta particle emitting radionuclides include Iodine-131, Yttrium-90, and Lutetium-177. Each isotope possesses unique characteristics, including half-life, specific beta energy levels, and potential gamma emissions. Iodine-131, for instance, has historically been used to treat thyroid disorders because the thyroid readily absorbs iodine. Yttrium-90 is frequently chosen for radioimmunotherapy applications, where antibodies labelled with beta radiation sources specifically bind to cancer cells. Lutetium-177 stands out due to its moderate beta energy, which promotes superior tissue targeting and minimises injury to surrounding non-cancerous tissues.
When these beta particle emitting radionuclides are bound to carefully designed radiopharmaceuticals, the resulting therapeutic agents exhibit improved selectivity for tumours. Moreover, modern imaging methods enable clinicians to track how the radionuclides move through the body in real-time, fine-tuning administration protocols. By calibrating treatment plans to meet individual patient needs, practitioners can promote patient safety while achieving robust therapeutic benefits. Progress in manufacture and supply has also contributed to the popularity of these radionuclides in the clinical setting, ensuring a consistent availability of suitable beta radiation emitters for therapeutic use.
Production and Preparation of Beta Particle Emitting Radiopharmaceuticals
A significant undertaking in therapeutic nuclear medicine is the consistent production of beta particle emitting radionuclides fit for medical treatments. Methods to procure these isotopes vary according to the specific element. Some rely on nuclear reactors, while others use accelerator-based systems. Once harvested, the raw radioactive material is chemically processed to produce radiopharmaceuticals with a strong affinity for tumour cells. Throughout this workflow, rigorous quality control is crucial to confirm that the final product meets stringent requirements for purity, safety, and efficacy.
Iodine-131, for example, commonly originates in nuclear reactors. Yttrium-90 often stems from Strontium-90 generators, with Strontium-90 decaying into Yttrium-90, which is subsequently extracted under sterile conditions. Lutetium-177 is typically created by bombarding Lutetium-176 or Ytterbium-176 with neutrons in a reactor. Each production process aims to produce radionuclides with the optimal half-life, decay energy, and specific activity for clinical use. These isotopes are then transported to specialist radiopharmacies, where they are joined to peptides, antibodies, or other carrier molecules.
Such labelling steps produce radiopharmaceuticals designed to excel in tissue targeting. Before full-scale clinical implementation, these new agents undergo rigorous preclinical testing, where scientists examine biodistribution, toxicity profiles, and therapeutic potential. Once regulatory bodies approve the formulations, they can be adopted for patient care. Advancements in protein engineering and nanotechnology have inspired the development of carrier molecules with enhanced specificity, further refining tissue targeting and minimising toxicity to healthy organs.
At every stage of production, patient safety is a fundamental priority. Strict handling protocols, specialised facilities, and controlled transportation methods are essential to protect staff from radiation hazards. Dosimetry calculations must be meticulous, ensuring the correct quantity of radioactive material is shipped and delivered. Waste management procedures also come under careful scrutiny to avoid environmental contamination. By maintaining these standards, healthcare practitioners can ensure that beta particle emitting radionuclides and associated radiopharmaceuticals reach the clinic in a manner that upholds patient safety and meets the clinical needs of therapeutic nuclear medicine.
Optimising Tissue Targeting and Treatment Efficacy
Therapies based on beta radiation depend heavily on precise tissue targeting. By coupling radioactive isotopes to carrier molecules that bind to tumour-specific receptors, clinicians reduce radiation doses to healthy tissues and heighten the chance of eradicating cancer cells. A prime example is peptide receptor radionuclide therapy (PRRT), which utilises somatostatin analogues labelled with beta radiation sources to address neuroendocrine tumours. These malignant cells often display high concentrations of somatostatin receptors, facilitating a highly selective approach to treatment and bolstering therapeutic outcomes.
An equally essential factor in tissue targeting is the molecular size, charge, and stability of the radiopharmaceuticals. Ideally, the agent should distribute swiftly in the body, reach the tumour site efficiently, and resist in vivo breakdown. Recent innovations in biotechnology have given rise to unique carrier systems, including nanoparticles, antibody fragments, and engineered proteins intended for specific receptor binding. By refining these biochemical and pharmacokinetic features, scientists and physicians can heighten tumour uptake, reduce off-target accumulation, and improve overall patient response.
The mechanism by which beta radiation hinders tumour growth involves the creation of direct and indirect DNA damage. When an optimal radiation dose is delivered to malignant tissues, the subsequent DNA damage often triggers cell death, halting further tumour progression. Nonetheless, some cancers may develop mechanisms that diminish their susceptibility to radiation, prompting a need for combined treatment regimens. Researchers are increasingly evaluating how chemotherapy, immunotherapy, and targeted agents may complement the use of beta particle emitting radionuclides. Personalised dosimetry tools, aided by advanced imaging, allow clinicians to adapt treatments to fit each patient’s unique disease profile, boosting the likelihood of a successful outcome. As techniques mature, the balanced focus on tissue targeting and patient safety cements the position of beta radiation therapies in the standard cancer treatment arsenal.
Patient Safety, Dosimetry, and Regulatory Considerations
Upholding patient safety is essential for any intervention utilising beta radiation sources. Accurate dose planning forms the bedrock of effective and cautious practice. Medical physicists use sophisticated modelling techniques to calculate how the administered radioactive agent will spread throughout the body and what levels of radiation each organ will receive. By matching these calculations with patient-specific factors—such as body mass, organ function, and tumour location—clinicians tailor treatment regimens that optimise disease control while minimising collateral damage.
Once the team establishes the correct dosage, carefully regulated protocols govern the administration of beta particle emitting radionuclides. These measures include shielded facilities to lessen radiation leakage, monitoring equipment to track ambient levels and professional training for healthcare staff on protective practices. Clear guidance is also shared with patients, advising them on how to minimise radiation exposure to loved ones following treatment. For instance, certain therapies involving Iodine-131 may require patients to observe temporary precautions to prevent radioactive iodine from affecting others. These diligent processes help clinicians preserve patient safety without undercutting therapeutic gains.
Regulatory authorities, both national and international, are central to assuring high standards in therapeutic nuclear medicine. They issue licences, inspect facilities, and provide guidelines designed to uphold ethical and scientific rigour. These agencies also remain vigilant over the production of radiopharmaceuticals, supervising procedures that guarantee purity, stability, and sterility. As scientific understanding evolves, guidelines adapt accordingly, ensuring that emerging forms of beta radiation therapy meet validated criteria for both efficacy and patient safety. Healthcare providers, researchers, and regulators communicate frequently, exchanging insights that foster evidence-based practice.
By extension, continuous quality improvement efforts, including peer review and accreditation programmes, support the integration of fresh research discoveries into practical settings. Treatment centres often partake in multi-centre collaborations, pooling patient data to evaluate long-term outcomes and address any side effects that arise. This collective commitment maintains patient safety at the highest standard and drives ongoing refinement of beta radiation approaches within therapeutic nuclear medicine.
Future Prospects and Conclusion
As cancer biology research progresses, beta particle emitting radionuclides are expected to remain valuable within therapeutic nuclear medicine. Ongoing improvements in generator technology, radiochemistry, and molecular biology feed into the development of next-generation radiopharmaceuticals with stronger tissue targeting properties. These enhanced agents promise more precise tumour localisation, reduced toxicity profiles, and new treatment protocols for difficult or resistant malignancies.
Combination therapies incorporating beta radiation alongside chemotherapy, targeted drugs, or immunotherapy also appear promising. Some tumours exhibit partial or complete resistance to a single treatment mode, prompting the need for collaborative strategies that strike cancer cells through multiple pathways. Additionally, personalised medicine endeavours exploit genomic profiling techniques to identify patient-specific tumour markers, guiding the selection of the most suitable radiolabelled agent. In parallel, dosimetry improvements build on advances in imaging technology to better predict how radionuclides will act in the body, further enhancing patient safety.
Another hurdle is ensuring an uninterrupted supply of robust beta particle emitting radionuclides, especially in areas with limited nuclear research infrastructure. Collaborative international efforts can mitigate such concerns by sharing resources, technical knowledge, and training opportunities. Education programmes for healthcare professionals, including nuclear medicine physicians, medical physicists, and radiopharmacists, ensure that expertise is both expanded and updated. By continually raising standards, practitioners can achieve more consistent outcomes and maintain the trust of patients and the wider public.
Conclusion
Selected beta particle emitting radionuclides remain integral elements of cancer treatment within therapeutic nuclear medicine. Their capability to deliver powerful beta radiation to tumour cells, combined with refined tissue targeting strategies and robust attention to patient safety, has propelled them to a prominent position in modern oncology. As innovative radiopharmaceuticals emerge and regulatory frameworks adapt, these therapies will likely evolve further, granting healthcare professionals a wider array of strategies to combat challenging malignancies. Through continued collaboration and research, clinicians can refine these interventions and extend their benefits, offering hope to patients worldwide.
Disclaimer
The content provided in this article is intended for informational and educational purposes only. It does not constitute medical, scientific, or professional advice and should not be relied upon as such. The article reflects current knowledge and practice in therapeutic nuclear medicine at the time of publication and may not account for future developments or individual clinical circumstances.
Open MedScience does not guarantee the accuracy, completeness, or timeliness of the information contained herein. Readers are encouraged to consult qualified healthcare professionals or regulatory bodies before making decisions based on the topics discussed, particularly those involving the use of radiopharmaceuticals or beta particle emitting radionuclides in clinical practice.
Any reference to specific radionuclides, procedures, or technologies is provided as general information and does not imply endorsement. Open MedScience disclaims any liability for outcomes arising from the application or interpretation of the content presented in this publication.
home » blog » radiotherapeutics »