Radioactive transformations, or nuclear decay processes, are a cornerstone of nuclear physics, underpinning our understanding of atomic nuclei and their inherent instability. This article provides an in-depth exploration of the various classes of radioactive transformations, looking into the mechanisms, characteristics, and implications of each. The article is divided into several sections, each dedicated to a specific type of radioactive decay, including alpha, beta, gamma, and other less common but significant transformations like electron capture and positron emission. Also, it examines the practical applications of these transformations in fields such as medicine, energy production, and scientific research, highlighting their importance in both theoretical and applied contexts.
Introduction to Radioactive Transformations
Radioactivity, the process by which unstable atomic nuclei lose energy by emitting radiation, is a fundamental phenomenon in nuclear physics. Discovered at the end of the 19th century by Henri Becquerel and further explored by scientists such as Marie Curie and Ernest Rutherford, radioactive decay has since been thoroughly studied and classified into several types. These transformations are crucial for understanding the stability of elements, the formation of new elements, and the vast array of applications that arise from the manipulation of radioactive materials.
This article will explore the various classes of radioactive transformations, providing detailed explanations of each type and discussing their relevance to both natural processes and human technology. The classes covered include alpha decay, beta decay (both beta-minus and beta-plus), gamma decay, and other significant but less common transformations such as electron capture, internal conversion, and neutron emission.
Alpha Decay: The Heaviest Emission
Alpha decay is one of the most common types of radioactive transformation, particularly among heavy elements. It involves the emission of an alpha particle, which consists of two protons and two neutrons, effectively a helium-4 nucleus. This type of decay occurs in very heavy elements such as uranium-238, thorium-232, and radium-226, where the large size and mass of the nucleus make it unstable.
Mechanism of Alpha Decay
The mechanism behind alpha decay is rooted in the balance of forces within the nucleus. The strong nuclear force binds protons and neutrons together, while the electromagnetic force causes repulsion between the positively charged protons. In very heavy nuclei, the repulsive electromagnetic force can become comparable to the attractive nuclear force, leading to instability. To reach a more stable state, the nucleus emits an alpha particle, which reduces its mass and charge.
Characteristics and Impact
Alpha particles are relatively heavy and carry a +2 charge, which makes them highly ionising but with low penetration power. They can be stopped by a sheet of paper or even human skin. However, if ingested or inhaled, alpha emitters can cause significant biological damage due to their high ionisation capability.
Applications of Alpha Decay
Alpha decay has practical applications in various fields. In medicine, alpha emitters are used in targeted radiotherapy, where they can destroy cancer cells with minimal damage to surrounding tissues. In space exploration, alpha decay is harnessed in radioisotope thermoelectric generators (RTGs), which provide long-lasting power for spacecraft by converting the heat released from alpha decay into electricity.
Beta Decay: Electron and Positron Emissions
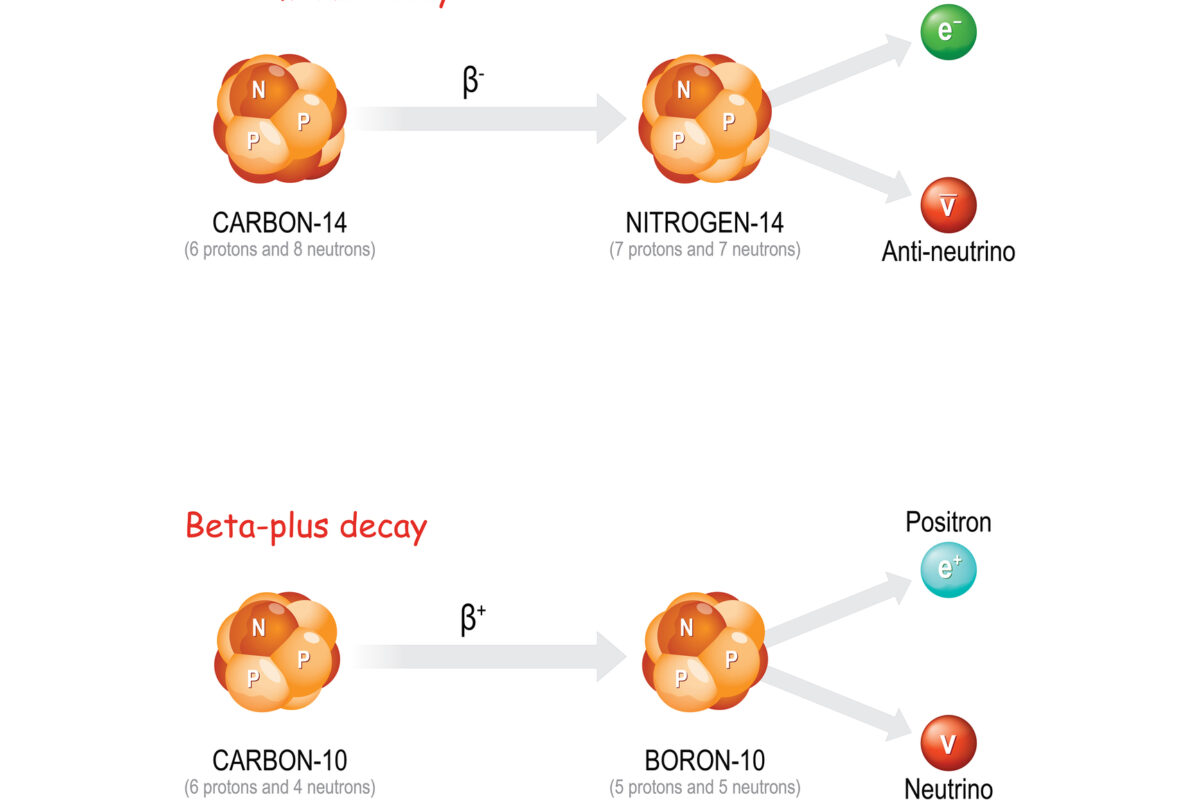
Beta-minus decay occurs when a neutron in the nucleus transforms into a proton, emitting an electron (beta particle) and an antineutrino. This transformation increases the atomic number of the element by one while the mass number remains unchanged. Beta-minus decay is common in isotopes where the neutron-to-proton ratio is too high for stability, such as carbon-14 and iodine-131.
Characteristics and Impact
The emitted beta particle is a high-energy electron, which is much lighter than an alpha particle and carries a single negative charge. Beta particles have greater penetration power than alpha particles but are less ionising. They can pass through several millimetres of human tissue, which makes them potentially hazardous, particularly in medical and industrial settings.
Applications of Beta-Minus Decay
Beta-minus decay has significant applications in medicine, particularly in diagnostic imaging and cancer treatment. Isotopes like technetium-99m are used in medical imaging to trace bodily functions. Beta-minus emitters are also used in radiotherapy to target and destroy cancerous tissues.
Beta-Plus Decay (Positron Emission)
Beta-plus decay, or positron emission, occurs when a proton in the nucleus transforms into a neutron, emitting a positron (the antimatter counterpart of an electron) and a neutrino. This process decreases the atomic number of the element by one, with the mass number remaining constant. Positron emission is common in isotopes with a proton-to-neutron ratio that is too high for stability, such as carbon-11 and fluorine-18.
Characteristics and Impact
Positrons are positively charged and, like beta particles, have a small mass. When a positron encounters an electron, they annihilate each other, producing gamma photons. This annihilation process is the basis for Positron Emission Tomography (PET) scans, a powerful imaging technique used in medical diagnostics.
Applications of Beta-Plus Decay
Positron emission is primarily used in medical imaging. PET scans allow for detailed imaging of metabolic processes in the body, aiding in the diagnosis and monitoring of diseases such as cancer and neurological disorders. Additionally, positron-emitting isotopes are used in research to study biochemical pathways in the body.
Gamma Decay: Electromagnetic Emission
Gamma decay involves the emission of gamma rays, which are high-energy photons, from an excited nucleus. This process usually follows alpha or beta decay, as the daughter nucleus often remains in an excited state after the initial transformation. Gamma decay allows the nucleus to release excess energy and reach a more stable configuration.
Mechanism of Gamma Decay
Unlike alpha and beta decay, gamma decay does not change the atomic number or mass number of the nucleus. Instead, it involves the release of electromagnetic radiation as the nucleus transitions from a higher energy state to a lower one. Gamma rays have no mass or charge but possess very high energy and penetration power.
Characteristics and Impact
Gamma rays are the most penetrating form of radiation, capable of passing through several centimetres of lead. They are less ionising than alpha or beta particles but can cause significant damage to living tissues and electronic equipment due to their deep penetration and high energy.
Applications of Gamma Decay
Gamma rays are widely used in medicine, industry, and research. In medicine, gamma emitters like cobalt-60 are used in radiotherapy to treat cancer. Gamma rays are also employed in sterilisation processes for medical equipment and food, as they can effectively kill bacteria and other pathogens. Additionally, gamma spectroscopy is a valuable tool in nuclear research for identifying and studying isotopes.
Electron Capture: An Alternative to Positron Emission
Electron capture is a process in which an atomic nucleus captures an inner orbital electron, typically from the K-shell, and combines it with a proton to form a neutron. This process decreases the atomic number by one, similar to positron emission, but without emitting a positron. Instead, it often results in the emission of an X-ray or an Auger electron as the atom reconfigures itself.
Mechanism of Electron Capture
During electron capture, the nucleus absorbs an electron from its inner orbitals, which leads to a reduction in the number of protons by one while the mass number remains constant. The captured electron combines with a proton to form a neutron and a neutrino, which is emitted from the nucleus. The vacancy left by the captured electron is filled by an electron from a higher energy level, which releases energy in the form of X-rays or Auger electrons.
Characteristics and Impact
Electron capture is often observed in isotopes where the proton-to-neutron ratio is too high for stability, but the energy difference between the parent and daughter nuclei is not sufficient to allow positron emission. The emitted X-rays or Auger electrons can cause ionisation and are detectable, making this process useful in analytical techniques.
Applications of Electron Capture
Electron capture is utilised in various scientific and medical applications. In nuclear medicine, isotopes like iodine-125 are used in brachytherapy, a form of radiotherapy. Electron capture is also exploited in X-ray fluorescence spectroscopy, a technique used for elemental analysis in materials science and chemistry.
Neutron Emission: A Rare but Significant Transformation
Neutron emission is a less common type of radioactive decay that involves the release of a neutron from an unstable nucleus. This type of decay typically occurs in very neutron-rich isotopes or in nuclei that have absorbed additional neutrons, as in the case of certain nuclear reactions.
Mechanism of Neutron Emission
In neutron emission, an unstable nucleus ejects a neutron to reduce its neutron-to-proton ratio and move towards a more stable configuration. This process does not change the atomic number of the element but decreases the mass number by one. Neutron emission can occur spontaneously or as a result of nuclear fission or fusion reactions.
Characteristics and Impact
Neutrons are neutral particles and, therefore, do not ionise atoms directly. However, they can penetrate deeply into materials and induce further nuclear reactions, making them particularly hazardous in certain contexts. Neutron radiation can activate other materials, turning them radioactive, and poses significant challenges in radiation protection.
Applications of Neutron Emission
Neutron sources are used in various fields, including materials science, nuclear research, and medicine. Neutron emission plays a critical role in nuclear reactors, where the emitted neutrons sustain the chain reaction necessary for energy production. In medicine, neutron radiation is used in certain types of cancer treatment, such as boron neutron capture therapy (BNCT), where neutrons are used to target and destroy tumour cells selectively.
Other Radioactive Transformations
Internal Conversion
Internal conversion is a process in which an excited nucleus transfers its energy directly to one of the atom’s inner electrons, causing the electron to be ejected from the atom. This process often competes with gamma decay and results in the emission of conversion electrons, which have discrete energies corresponding to the difference between the nuclear energy levels.
Double Beta Decay
Double beta decay is an extremely rare process where two neutrons in a nucleus are simultaneously converted into two protons, emitting two beta particles and two antineutrinos. There is also a theoretical variant known as neutrinoless double beta decay, where no neutrinos are emitted, which, if observed, would have profound implications for our understanding of particle physics and the nature of neutrinos.
Spontaneous Fission
Spontaneous fission is a form of radioactive decay in which a heavy nucleus splits into two smaller nuclei along with a few neutrons and a significant amount of energy. This process is most common in very heavy elements like uranium-238 and californium-252 and is the principle behind the operation of nuclear reactors and atomic bombs.
Applications of Radioactive Transformations
Medical Applications
Radioactive transformations have revolutionised medicine, particularly in the fields of diagnostic imaging and cancer treatment. Techniques such as PET scans, gamma cameras, and radiotherapy rely on the precise control and application of radioactive decay processes. Radioisotopes like iodine-131, technetium-99m, and cobalt-60 are integral to these technologies, providing invaluable tools for diagnosis and treatment.
Energy Production
Nuclear energy is one of the most significant applications of radioactive transformations. Nuclear reactors harness the energy released from fission reactions, primarily involving uranium and plutonium isotopes, to generate electricity. The controlled chain reactions in reactors rely on the principles of neutron emission and fission, highlighting the importance of understanding radioactive decay processes.
Scientific Research
Radioactive transformations are also fundamental to scientific research. Radiocarbon dating, which uses the decay of carbon-14, allows archaeologists and geologists to determine the age of ancient artefacts and geological formations. Additionally, radioactive tracers are used in various fields of science to study processes ranging from chemical reactions to biological pathways.
Industrial Applications
In industry, radioactive materials are used for a variety of purposes, including material testing, quality control, and sterilisation. Gamma rays are employed in non-destructive testing to inspect the integrity of materials and structures. Radioisotopes are also used in smoke detectors, where the ionising radiation from americium-241 helps to detect smoke particles.
Conclusion
Radioactive transformations are diverse and complex processes that play a crucial role in both natural phenomena and human technology. Understanding the various classes of radioactive decay—alpha, beta, gamma, neutron emission, electron capture, and others—is essential for harnessing their potential benefits while mitigating their risks. From powering spacecraft to diagnosing diseases, the applications of radioactive decay are vast and varied, underscoring the importance of continued research and development in this field. As our understanding of these processes deepens, so too will our ability to innovate and apply them in ways that benefit society and expand our knowledge of the universe.
Disclaimer
The content provided in this article, Understanding Radioactive Transformations to Nuclear Decay Processes, is intended for informational and educational purposes only. While efforts have been made to ensure the scientific accuracy of the material presented, Open MedScience does not guarantee the completeness, reliability, or suitability of the content for any particular purpose.
This article does not constitute professional advice or services in the fields of nuclear physics, medicine, or engineering. Readers are encouraged to consult qualified professionals or authoritative sources before making decisions or taking action based on the information provided.
Open MedScience shall not be held liable for any loss, damage, or injury resulting from the use or misuse of the information contained in this publication. The inclusion of specific examples or references to medical, industrial, or research applications does not imply endorsement or recommendation by Open MedScience.
Any links or references to external sites or third-party materials are provided for convenience only and do not constitute an endorsement or responsibility for their content.
All scientific concepts discussed reflect current understanding as of the publication date and may evolve with ongoing research and developments in the field.
home » blog » medical physics »