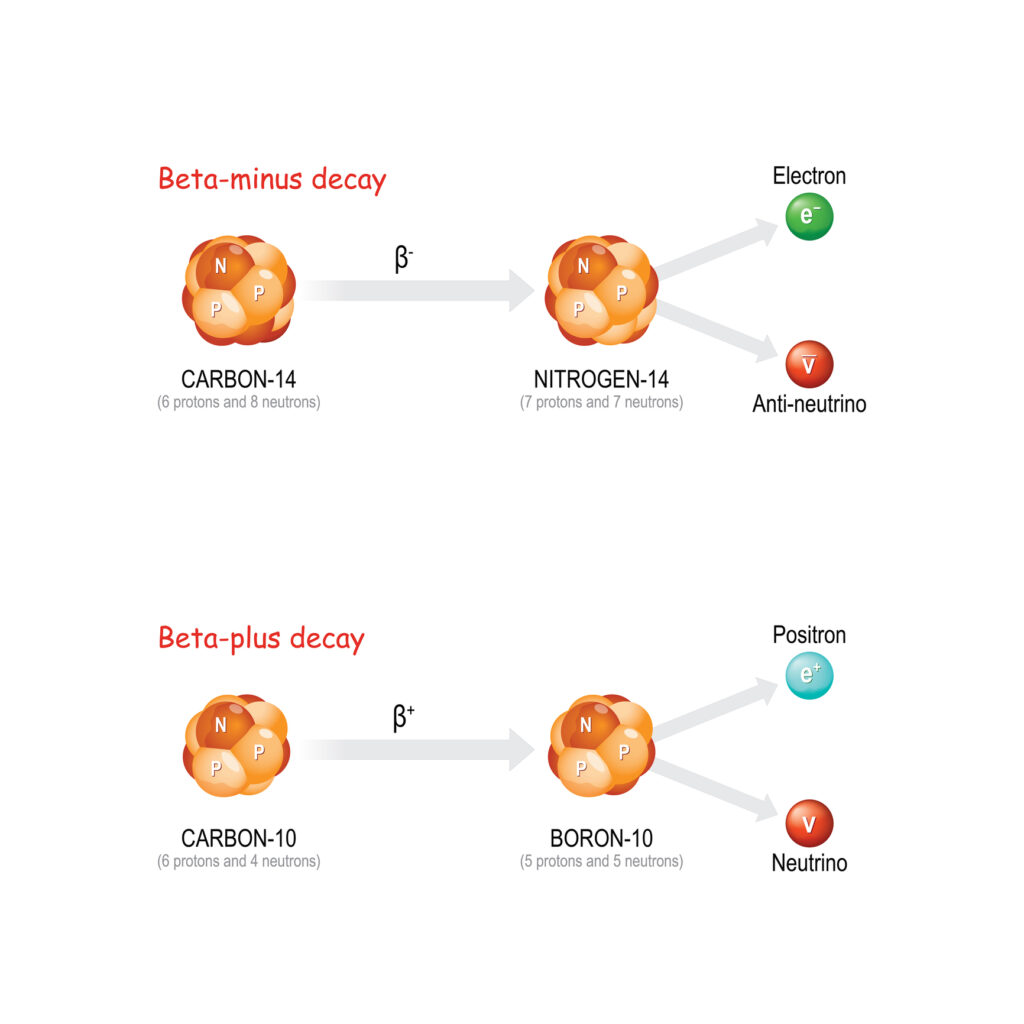
Electron capture is a type of radioactive decay in which an atomic nucleus absorbs an inner atomic electron. This process leads to the transformation of a proton into a neutron, resulting in a change of the element. Commonly occurring in isotopes with a high proton-to-neutron ratio, electron capture plays a crucial role in nuclear physics and various scientific applications, including astrophysics and medical imaging.
Introduction to Electron Capture
Electron capture in radioactive decay, also known as inverse beta decay, is a fundamental nuclear process. Discovered in the mid-20th century, it provides insights into the stability of atoms and the forces that govern nuclear interactions. This process is significant in elements with a large number of protons, where the strong nuclear force is counterbalanced by electrostatic repulsion among protons.
The Mechanism of Electron Capture
Electron capture in radioactive decay involves an electron from the innermost electron shell, usually the K-shell, being captured by the nucleus. This electron combines with a proton to form a neutron and a neutrino. The neutrino is emitted from the nucleus, conserving energy and momentum. The overall reaction can be summarised as:
p+ + e− → n0 + νe
Here, p+ represents a proton, e− an electron, n0 a neutron, and νe an electron neutrino.
Energy Considerations
For electron capture to occur, the energy difference between the initial and final states must be positive. The mass of the resulting atom must be less than the original atom’s mass plus the electron’s mass. This energy difference is typically manifested as the captured electron’s binding energy and the emitted neutrino’s kinetic energy.
Conditions for Electron Capture
Nuclear Stability
Electron capture is more likely in nuclei with a high proton-to-neutron ratio. Such nuclei tend to be unstable and seek stability through various decay processes, including electron capture. This process reduces the number of protons and increases the number of neutrons, moving the nucleus towards a more stable configuration.
Competing Processes
Electron capture competes with positron emission, another form of decay in proton-rich nuclei. While both processes reduce the number of protons, the choice between them depends on the available energy and the mass difference between the initial and final states. Both processes can occur in certain isotopes, but electron capture becomes more probable when positron emission is energetically unfavourable.
Examples of Electron Capture
Carbon-11
Carbon-11, a radioactive isotope of carbon, undergoes electron capture to form boron-11. This isotope is widely used in medical imaging in positron emission tomography (PET).
11C + e− → 11B + νe
Beryllium-7
Beryllium-7 is another example, capturing an electron to become lithium-7. This process is observed in the context of solar neutrinos and has implications for understanding solar processes.
7Be + e− → 7Li + νe
Potassium-40
Potassium-40 undergoes electron capture to form argon-40. This isotope is significant in geological dating methods, such as argon-argon dating.
40K + e− → 40Ar + νe
Detection and Measurement
Neutrino Emission
The detection of neutrinos emitted during electron capture is challenging due to their weak interaction with matter. Specialised detectors, such as those used in neutrino observatories, are employed to observe these elusive particles.
X-Ray Emission
When an inner electron is captured, the resulting vacancy in the electron shell is filled by an outer electron, emitting X-rays in the process. These X-rays can be detected and measured to study electron capture events.
Applications of Electron Capture
Astrophysics
Electron capture plays a pivotal role in the life cycles of stars. During the late stages of stellar evolution, electron capture affects the core composition of massive stars, influencing their collapse and subsequent supernova events.
Medical Imaging
In medical imaging, isotopes that undergo electron capture, such as iodine-123, are used in diagnostic procedures. These isotopes help create detailed images of organs and tissues, aiding in the diagnosis of various conditions.
Nuclear Physics Research
Electron capture is utilised in nuclear physics research to study the properties of neutrinos and the fundamental forces within the nucleus. Experiments involving electron capture contribute to our understanding of weak interactions and the behaviour of subatomic particles.
Theoretical Implications
Weak Interaction
Electron capture is governed by the weak nuclear force, one of the four fundamental forces of nature. Electron capture studies enhance our understanding of weak interactions and their role in particle physics.
Neutrino Mass
Electron capture investigations provide insights into neutrinos’ properties, including their masses. Precise measurements of electron capture rates and neutrino emissions help constrain theoretical models of neutrino physics.
Challenges and Future Directions
Experimental Challenges
The detection of neutrinos and precise measurement of electron capture events pose significant experimental challenges. Advances in detector technology and data analysis methods are crucial for overcoming these hurdles.
Theoretical Models
Developing accurate theoretical models of electron capture requires a deep understanding of nuclear structure and the weak interaction. Ongoing research aims to refine these models and improve our predictive capabilities.
Conclusion
Electron capture is a fundamental nuclear process with wide-ranging implications in physics, astrophysics, and medical imaging. By transforming protons into neutrons, it plays a key role in the stability and behaviour of atomic nuclei. Through continued research and technological advancements, our understanding of electron capture and its applications will continue to grow, shedding light on the intricate workings of the universe.
Disclaimer
The content provided in this article, Unlocking the Secrets of Electron Capture: A Journey Through Nuclear Transformations, is intended for informational and educational purposes only. While every effort has been made to ensure the accuracy and reliability of the information presented, Open MedScience makes no guarantees regarding its completeness, timeliness, or applicability to specific circumstances.
This article does not constitute professional advice in the fields of nuclear physics, medical imaging, or any related discipline. Readers are advised to consult qualified experts or peer-reviewed sources for guidance on scientific, medical, or technical matters.
Open MedScience shall not be held responsible for any errors, omissions, or consequences arising from the use or misuse of the information contained herein. The views expressed in this article are those of the authors and do not necessarily reflect the position of Open MedScience or its affiliates.
Any references to scientific data, processes, or isotopes are for illustrative purposes and may be subject to change as research in these fields progresses.
home » blog » medical physics »