This article explores nuclear chemistry, a branch of chemistry that studies the changes in atomic nuclei. The discussion encompasses the basics of nuclear reactions, radiation types, nuclear chemistry applications in various fields, and the safety and ethical considerations associated with nuclear technology. The article is structured into several sections to systematically cover the theoretical aspects of nuclear chemistry, including its practical applications and future prospects.
Introduction to Nuclear Chemistry
Nuclear chemistry is the branch of chemistry that deals with nuclear reactions and the chemical applications of nuclear processes. This field focuses on the interactions and transformations in the nuclei of atoms, distinct from conventional chemistry, which concerns itself with electron shell interactions in chemical reactions. Nuclear chemistry examines processes such as radioactive decay, nuclear fission and fusion, and isotopic labelling, playing a crucial role in energy generation, medical diagnostics and treatment, and industrial applications.
The scope of nuclear chemistry extends beyond simple atomic theory, especially into atomic energy and radiation mechanisms. It encompasses the study of both stable and unstable isotopes, exploring how unstable nuclei release energy during decay. Nuclear chemists work to harness this energy for a variety of applications, pushing the boundaries of science to innovate in fields like medicine, where isotopic techniques are used for imaging and therapy, and energy, where nuclear reactions provide a substantial portion of the world’s electricity.
Historical Development
The foundations of nuclear chemistry were laid in the late 19th and early 20th centuries, a period marked by pioneering discoveries that expanded our understanding of atomic structure. In 1896, Henri Becquerel accidentally discovered radioactivity, observing that uranium salts emitted rays that could penetrate solid matter and fog photographic plates. Marie Curie furthered this work, coining the term “radioactivity” and discovering the elements polonium and radium. Her groundbreaking research laid the groundwork for the use of radioactive isotopes and radiation in treatment and diagnostics.
Another significant milestone was James Chadwick’s identification of the neutron in 1932. This solved the puzzle of atomic mass and led to the discovery of isotopes, thereby enhancing the depth and range of applications in nuclear chemistry. This discovery was crucial for the development of nuclear fission, as neutrons proved essential in sustaining nuclear chain reactions.
The conceptual leap from laboratory-scale nuclear reactions to practical applications occurred in the mid-20th century. The development of the atomic bomb during World War II under the Manhattan Project demonstrated the vast energy potential of nuclear fission. Post-war, the focus shifted to peaceful applications, leading to the development of nuclear power plants. The first controlled nuclear chain reaction initiated by Enrico Fermi in 1942 laid the theoretical and practical foundations for nuclear power generation.
Since then, nuclear chemistry has evolved into a field with profound implications for various sectors. In medicine, techniques such as positron emission tomography (PET) scans have revolutionised diagnostic imaging, allowing for detailed and non-invasive observation of metabolic processes in real-time. In industry, nuclear techniques are used in materials science, archaeology, and environmental science to analyse compositions and age materials.
Today, nuclear chemistry remains a dynamic field of scientific inquiry and technological innovation. Researchers continue to explore the potentials of nuclear processes, seeking safer and more efficient ways to harness nuclear energy, develop new medical imaging and treatment options, and mitigate the environmental impact of nuclear waste. The historical development of nuclear chemistry reflects its expansive scope and application and its crucial role in advancing human knowledge and capability.
Basics of Nuclear Reactions
Nuclear reactions involve changes in the composition of an atom’s nucleus and typically result in the conversion of elements into other elements and the emission of radiation. These reactions are crucial to nuclear power, medical imaging, and astrophysics, among other fields. Understanding the different types of nuclear reactions, how to balance nuclear equations, and the energy considerations involved are fundamental to nuclear chemistry.
Types of Nuclear Reactions
Fission
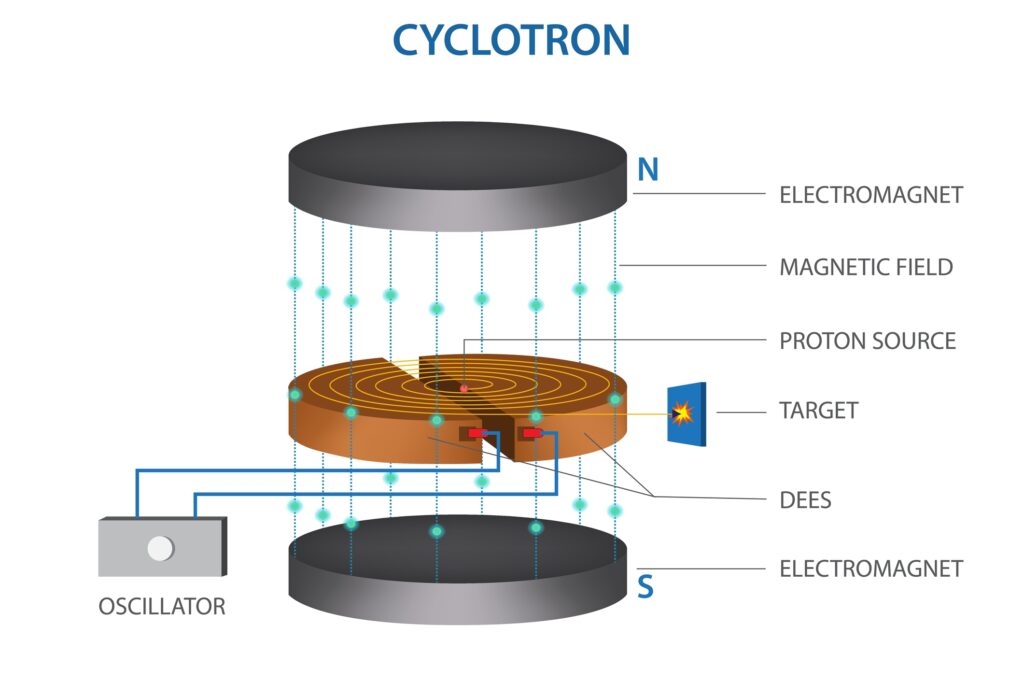
Nuclear fission is a type of nuclear reaction in which the nucleus of an atom splits into two or more smaller nuclei, along with a few neutrons and a large amount of energy. This reaction can be spontaneous, but more often, it is induced by the interaction of an element (usually uranium-235 or plutonium-239) with a neutron. The fission process releases a significant amount of energy, which is explained by Einstein’s equation E=mc2, where E is energy, m is mass, and c is the speed of light in a vacuum.
The process of nuclear fission typically produces two fission fragments of unequal mass, and the sum of the masses of these fragments is less than the original mass. This missing mass (mass defect) has been converted into energy. For example, when uranium-235 undergoes fission after capturing a neutron, it might split into barium-141 and krypton-92, releasing three neutrons and a large amount of energy:
235U + 1n → 141Ba + 92Kr + 3 1n
The newly released neutrons may trigger further fission reactions, potentially leading to a chain reaction if sustained. This chain reaction is the principle behind nuclear reactors and atomic bombs.
Fusion
Nuclear fusion is the process where two light atomic nuclei combine to form a heavier nucleus, releasing energy in the process. Fusion is the reaction that powers the sun and other stars, where hydrogen nuclei fuse under extreme pressure and temperature to form helium.
The fusion of deuterium and tritium, isotopes of hydrogen, is one of the most researched fusion reactions due to its relatively high energy yield:
2H + 3H → 4He + 1n
This reaction produces a helium nucleus and a neutron, releasing around 17.6 MeV of energy, considerably more than most fission reactions. The challenge with harnessing fusion energy on Earth lies in achieving and maintaining the extremely high temperatures and pressures needed to initiate and sustain the reaction.
Balancing Nuclear Equations
Balancing nuclear equations involves ensuring the conservation of both mass and charge before and after a reaction. In nuclear reactions, this means ensuring that the sum of atomic numbers (protons) and the sum of mass numbers (protons and neutrons) are equal on both sides of the equation. For instance, in the fission of uranium-235:
235U + 1n → 141Ba + 92Kr + 3 1n
Here, the sum of the mass numbers on both sides is 236 (235 from uranium and 1 from the neutron), which equals the sum of the mass numbers of the products (141 from barium, 92 from krypton, and 3 from the neutrons). The atomic numbers are also balanced, with 92 (uranium) on the left and the sum of the atomic numbers of barium (56) and krypton (36) plus the neutrons (0) on the right.
Energy Considerations in Nuclear Reactions
The energy released in nuclear reactions is significantly greater than in chemical reactions because the binding energy that holds the nucleus together is much larger than the energy that holds electrons in atoms. The binding energy per nucleon (average energy needed to remove a nucleon from the nucleus) varies across different elements, generally increasing with atomic number up to iron and decreasing thereafter. This explains why energy can be released by both splitting heavy nuclei (fission) and combining light nuclei (fusion).
In practical terms, the amount of energy released in a nuclear reaction can be calculated using the mass defect and Einstein’s equation E=mc2. This relationship provides the theoretical foundation for understanding the immense power of nuclear energy and its potential both as a weapon and as a sustainable energy source.
Therefore, nuclear reactions are a cornerstone of nuclear chemistry, with profound implications for energy, medicine, and the fundamental understanding of matter. To mastermind these reactions, from theoretical calculations to practical applications, remains a pivotal challenge and opportunity in science.
Types of Radiation
In nuclear chemistry, radiation refers to the energy particles or waves emitted from the nucleus of an unstable atom as it undergoes radioactive decay. The main types of radiation are alpha, beta, and gamma, each with unique properties and effects. Understanding these types of radiation, their properties, how they can be detected, and their sequences in decay series is essential for applications ranging from medical treatments to nuclear energy management.
Alpha, Beta, and Gamma Radiation
Alpha Radiation
Alpha radiation consists of alpha particles, each composed of two protons and two neutrons, making them essentially helium nuclei. During radioactive decay, these particles are emitted from the nuclei of very heavy elements, such as uranium or radium. Alpha particles are relatively massive and carry a double positive charge. Due to their size and charge, alpha particles have low penetration power and can be stopped by a sheet of paper or a few centimetres of air. However, despite their low penetration, alpha particles are highly ionising within their short range, meaning they can cause significant damage to materials they directly interact with, including human tissue.
Beta Radiation
Beta radiation involves the emission of beta particles, which are high-energy, high-speed electrons or positrons ejected from the nucleus of a radioactive atom. Beta particles are much smaller than alpha particles and carry a single electron charge, either negative or positive. They have greater penetration power than alpha particles, capable of passing through paper but generally stopped by a layer of clothing or a few millimetres of a substance like aluminium. Beta radiation moderately ionises and is capable of penetrating living tissue and causing ionisation along its path, which can lead to cellular damage.
Gamma Radiation
Gamma radiation consists of gamma rays, which are high-energy electromagnetic waves emitted from the nucleus. Unlike alpha and beta radiation, gamma rays are not particles and thus do not have mass or charge. They have extremely high penetration power and can pass through several centimetres of lead or metres of concrete. Gamma rays are the least ionising of the three types of radiation, but due to their deep penetration, they pose a serious internal and external health hazard.
Properties and Detection of Radiation
The properties of radiation, such as its energy, penetration power, and ionising ability, will determine its interaction with matter and its detection. Detecting radiation typically involves capturing the changes it induces in a medium. Alpha particles, for example, can be detected through their ionisation of air in an ionisation chamber or their light emission in a scintillator. Beta particles are often detected by their ability to cause excitation and subsequent photon emission in phosphor-based detectors or their direct ionisation effects in semiconductor devices. Gamma rays are usually detected via their penetration and interaction in dense materials, which can cause scintillation or excite electrons in semiconductor detectors.
Radiation detectors are crucial for a variety of applications, including medical imaging, environmental monitoring, nuclear power plant operations, and radiation therapy. These detectors help measure the intensity and type of radiation and ensure safety by monitoring exposure levels and maintaining them within safe limits.
Radioactive Decay Series
A radioactive decay series is a sequence of decay processes by which radioactive isotopes are transmuted into more stable isotopes until a stable isotope is formed. These series are important in understanding the lifecycle of radioactive elements and in applications such as dating rocks and archaeological artefacts.
For instance, the uranium-238 decay series, one of the most significant decay chains, includes multiple steps involving alpha and beta decay. It leads from uranium-238 through a series of intermediate isotopes to lead-206, a stable isotope. Each step in the decay series is characterised by a distinct half-life and decay mode, influencing the isotopic composition of natural materials and the environment’s distribution of particles and energy.
Understanding radioactive decay series is crucial for managing radioactive waste, optimising nuclear medicine procedures, and conducting environmental assessments and geological age determinations.
In summary, the study of different types of radiation and their properties is a key area in nuclear chemistry, critical for harnessing nuclear technology for beneficial uses while protecting health and the environment from potential risks.
Radioisotopes and Their Applications
Radioisotopes, isotopes of elements that emit radiation as they decay, are used extensively across various fields, including medicine, industry, agriculture, and environmental science. Their unique properties make them invaluable tools for diagnostics, treatment, and tracing processes in complex systems. This exploration of radioisotopes will cover their critical applications in medical diagnostics and treatment, industrial operations, and agricultural and environmental monitoring.
Medical Uses
Diagnostic Imaging
Radioisotopes are used primarily in medical diagnostics because they can provide detailed images of the body’s internal structures and functions. Techniques such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) rely on radioactive tracers that emit gamma rays detectable by specialised cameras. These tracers are typically linked to substances that naturally accumulate in specific tissues, such as glucose analogues or dyes, which allows for targeted imaging.
For example, Fluorodeoxyglucose (FDG), a radioactive glucose compound used in PET scanning, highlights areas of high metabolic activity, often indicating tumour growth. This method provides crucial information on tumour location, size, and activity, significantly improving the accuracy of diagnoses and the effectiveness of treatment planning.
Cancer Treatment
In cancer treatment, radioisotopes are used for both external beam radiotherapy and internal treatment (brachytherapy). External beam therapy often involves gamma rays from sources like cobalt-60, aimed precisely at cancerous tissues. This treatment focuses high-energy waves directly at the tumour, minimising damage to surrounding healthy tissues.
Brachytherapy, on the other hand, involves placing radioactive sources directly within or near the tumour. This provides a high radiation dose to the tumour while reducing the radiation exposure in the surrounding healthy tissues. Radioisotopes such as iodine-131, cesium-131, and iridium-192 are commonly used, depending on the tumor type and location.
Industrial Applications
Tracers
Radioisotopes are also indispensable in various industrial applications, especially as tracers in the study of processes. Researchers can track their path and dispersion using sensitive detection equipment by adding radioactive isotopes to materials. This method is extensively used in the oil and gas industry to trace the flow of oil in wells, in chemical manufacturing to study reaction processes, and in hydrology to trace water sources and pollution pathways.
For example, tritium (hydrogen-3) is used to trace water movements, whereas carbon-14 can trace organic compounds in ecosystems and industrial systems. These applications are crucial for optimising processes, ensuring safety, and minimising environmental impact.
Energy Production
Radioisotopes play a critical role in the energy sector, particularly in nuclear energy production. Neutron sources like californium-252 are used to start up nuclear reactors and measure sodium levels in coolant systems, a crucial aspect of reactor safety. Additionally, gamma radiography with isotopes like iridium-192 and cobalt-60 is used for the non-destructive testing of materials used in constructing and maintaining nuclear reactors, pipelines, and other infrastructure.
Agricultural and Environmental Applications
Agricultural Uses
In agriculture, radioisotopes contribute to improving crop varieties and studying soil conditions. Radiation-induced mutation breeding has been beneficial for developing new plant varieties with desirable traits such as increased yield, pest resistance, and drought tolerance. Phosphorus-32 and sulfur-35, as radioactive tracers, help in studying the uptake of fertilisers by plants, which assists in optimising fertiliser use and improving crop production efficiency.
Environmental Applications
Environmental science benefits from the use of radioisotopes in studying pollution dispersion and tracing environmental changes. Carbon-14 and tritium are particularly useful in dating water and understanding geological and hydrological processes over long periods. Radioisotopes also play a role in monitoring atmospheric pollutants and in the assessment of sediment transport and erosion in rivers and oceans.
Therefore, radioisotopes are powerful tools across diverse sectors, offering unique insights through their radioactive properties. Whether they are used to diagnose and treat diseases, enhance industrial and energy processes, improve agricultural practices, or monitor environmental changes, the applications of radioisotopes are vast and vital. Their ability to precisely target and trace processes makes them indispensable in modern science and technology.
Nuclear Energy Production
Nuclear energy remains a cornerstone in the quest for sustainable and high-capacity energy sources, given its capability to produce a significant amount of electricity without the direct emission of greenhouse gases. Understanding the functioning of nuclear reactors, the nuclear fuel cycle, and the advantages and disadvantages associated with nuclear energy is essential for a balanced view of this powerful energy source.
Functioning of Nuclear Reactors
Nuclear reactors are the heart of a nuclear power plant, where nuclear fission reactions are initiated, controlled, and sustained to produce heat. This heat is used to generate steam that drives turbines, which in turn generate electricity. The most common type of reactor is the Light Water Reactor (LWR), which includes both Pressurised Water Reactors (PWRs) and Boiling Water Reactors (BWRs).
In a PWR, the water used as a coolant is kept under high pressure to prevent it from boiling. This hot pressurised water passes through a heat exchanger, where it heats a secondary water line to create steam. In contrast, in a BWR, the water boiled by the nuclear reaction directly turns the turbine. The core of a nuclear reactor contains fuel rods, typically made of uranium, which are arranged to facilitate the controlled nuclear chain reaction. Control rods made of materials that absorb neutrons, such as boron or cadmium, are inserted or withdrawn to regulate the reaction rate.
Nuclear Fuel Cycle
The nuclear fuel cycle describes the lifecycle of nuclear fuel from its creation to disposal. It begins with the mining and milling of uranium ore, followed by the conversion of uranium into a gas, which is then enriched to increase the concentration of uranium-235. This enriched uranium is fabricated into fuel rods, which are then ready to be used in a reactor.
After spending typically three to five years in the reactor, the fuel becomes less efficient and must be replaced. This spent fuel is highly radioactive and generates a significant amount of heat. Therefore, it is cooled in spent fuel pools at the reactor site before potentially being reprocessed or moved to dry cask storage. Reprocessing can recover fissile materials to be reused as nuclear fuel, but it is controversial because of proliferation risks and environmental concerns.
Advantages and Disadvantages of Nuclear Energy
Advantages
One of the most significant advantages of nuclear energy is its capacity to generate large amounts of electricity with relatively small amounts of fuel without emitting carbon dioxide during operation. This makes it an attractive option for reducing greenhouse gas emissions and combating climate change. Additionally, nuclear power plants have a high capacity factor, meaning they produce electricity at or near their maximum capacity more than 90% of the time, providing a stable, reliable source of energy.
Disadvantages
However, nuclear energy comes with notable disadvantages. While the risk of accidents is statistically low, they can have catastrophic consequences, as evidenced by the Chernobyl and Fukushima disasters. These incidents highlight the potential for widespread environmental contamination and severe public health impacts. Additionally, the management of radioactive waste remains a significant challenge. High-level waste requires secure, long-term storage solutions to protect human health and the environment, as it remains hazardous for thousands of years.
Another disadvantage is the high cost of building, maintaining, and decommissioning nuclear power plants, coupled with long construction times, which can lead to significant financial risks and economic burdens.
Nuclear energy offers a powerful, low-carbon solution to global energy demands but is accompanied by considerable risks and challenges, particularly related to safety, waste management, and economic viability. The future of nuclear energy likely depends on advances in reactor technology, including the development of safer, more efficient, and less wasteful reactors such as small modular reactors and next-generation designs.
Safety and Regulation in Nuclear Chemistry
Safety and regulation are paramount in nuclear chemistry due to the potential hazards associated with radiation and radioactive materials. Ensuring the safe handling, use, and disposal of nuclear materials involves comprehensive measures, strict regulatory frameworks, and stringent standards both at national and international levels. This article will review radiation safety measures, nuclear activity regulatory frameworks, and waste management and disposal protocols.
Radiation Safety Measures
Safety in environments where radiation is used or produced revolves around three fundamental principles: time, distance, and shielding. Minimising the time spent near radioactive sources, maximising the distance from the source, and utilising appropriate shielding materials are key strategies to reduce radiation exposure.
Shielding involves using materials that absorb or block radiation, such as lead for gamma rays or concrete and water for neutron and gamma radiation. Radiation detection and monitoring devices, like Geiger counters, dosimeters, and ionisation chambers, are crucial for providing real-time data on radiation levels and ensuring they remain below safety thresholds.
Moreover, safety measures include rigorous training for personnel to handle radioactive materials and emergency response protocols safely. To protect workers from radiation risks, personal protective equipment (PPE), such as lead aprons or radiation badges, is mandatory in high-exposure areas.
Regulatory Frameworks and International Standards
National regulatory bodies oversee nuclear chemistry regulation, such as the Nuclear Regulatory Commission (NRC) in the United States. The NRC sets standards and guidelines for nuclear safety, licensing, and waste management and ensures that nuclear facilities operate within safety norms to protect the public and the environment.
Internationally, the International Atomic Energy Agency (IAEA) plays a critical role in setting global safety standards and providing guidelines for nuclear safety. The IAEA helps coordinate international efforts to strengthen nuclear safety protocols and assists countries in adhering to safety standards. The Agency also facilitates international treaties and conventions, such as the Convention on Nuclear Safety and the Joint Convention on the Safety of Spent Fuel Management and the Safety of Radioactive Waste Management, which commits member states to maintain high standards of nuclear safety and waste management.
Waste Management and Disposal
The management and disposal of nuclear waste are critical components of nuclear safety. They involve safely handling, processing, and disposing of radioactive waste materials. Nuclear waste is categorised into low-level waste (LLW), intermediate-level waste (ILW), and high-level waste (HLW), each requiring different disposal strategies.
Low-level waste, which includes items like contaminated gloves and tools, can often be disposed of in near-surface disposal facilities after treatment, which may consist of compaction or incineration. Intermediate-level waste, which might include resins, chemical sludge, and metal nuclear fuel cladding, typically requires more substantial shielding and may be solidified in concrete or bitumen before disposal in deep geological facilities.
High-level waste, primarily spent nuclear fuel and materials from reprocessing such fuel, presents the most significant challenge due to its high radioactivity and long-lived isotopes. This waste is usually cooled underwater or in dry casks until it is no longer thermally hazardous. It is then buried in deep geological repositories, engineered to contain and isolate radioactive material for thousands of years until it decays to safe levels.
The safe practice of nuclear chemistry underpins the protection of public health and the environment and the sustainability of nuclear technology as a vital energy resource. Effective radiation safety measures, robust regulatory frameworks, and meticulous waste management and disposal strategies are essential to managing the risks associated with nuclear materials and ensuring a safe future for nuclear technology.
Ethical Considerations and Public Perception of Nuclear Technology
The use of nuclear technology, whether for energy production, medical applications, or national security, presents a range of ethical considerations and significantly impacts public perception. The ethical debates often revolve around the implications for public health and the environment, the responsibilities of nuclear non-proliferation, and the overarching need for arms control. These factors influence how nuclear technology is viewed and regulated, affecting its development and application globally.
Ethical Issues in the Use of Nuclear Technology
Ethical considerations in nuclear technology often focus on the potential risks versus the benefits. For instance, while nuclear power plants provide substantial amounts of low-carbon energy, which is crucial in the fight against climate change, they also pose risks of catastrophic accidents, as evidenced by the Chernobyl and Fukushima disasters. These incidents raise ethical questions about risk management, the acceptable level of risk, and the equitable distribution of benefits and risks among different communities and generations.
In medical uses of nuclear technology, such as radiotherapy and radiopharmacology, ethical concerns include patient safety, informed consent, and the right to access advanced treatments. Balancing the potential life-saving benefits against the risks of exposure to radiation is a continuous ethical challenge in healthcare.
Moreover, the ethical use of nuclear technology extends to how countries manage nuclear waste. The long-term storage and disposal of radioactive waste pose risks for current and future generations, raising issues about intergenerational equity and the rights of future humans to a safe and clean environment.
Impact on Public Health and Environment
The impact of nuclear technology on public health and the environment is a significant area of concern. Radioactive leaks and nuclear accidents can have devastating effects on human health, causing acute radiation sickness, increased cancer risks, and long-term genetic damage. The environmental impact includes the contamination of land and water, which can last for thousands of years, affecting ecosystems and human settlements alike.
The ethical implications of these impacts involve the duty to prevent harm and mitigate environmental damage where it occurs. This includes investing in safer technologies, improving emergency preparedness, and ensuring that communities living near nuclear facilities are informed and protected as much as possible.
Nuclear Non-Proliferation and Arms Control
Nuclear non-proliferation and arms control are perhaps nuclear technology’s most significant ethical arenas. The proliferation of nuclear weapons poses a substantial threat to global security. Ethical concerns arise regarding the right of nations to pursue nuclear technology for peaceful purposes while ensuring that this technology does not contribute to the development of nuclear arms.
The Treaty on the Non-Proliferation of Nuclear Weapons (NPT) aims to prevent the spread of nuclear weapons and weapon technology, to promote cooperation in the peaceful uses of nuclear energy, and to further the goal of achieving nuclear disarmament. Ethical issues here include the responsibilities of nuclear-armed states to disarm and the rights of non-nuclear-armed states to access nuclear technology for energy.
Moreover, the ethical responsibility to avoid nuclear war and to work towards disarmament involves complex negotiations and trust-building measures among countries. The maintenance of peace and security, the prevention of arms races, and the ethical imperative to protect human life on a large scale are central to discussions of nuclear arms control.
The ethical considerations and public perceptions surrounding nuclear technology are complex and multifaceted. They encompass a broad spectrum of issues, from individual and public health to global security and environmental sustainability. Addressing these ethical challenges requires a nuanced understanding of both the scientific realities and the moral implications, guiding responsible and equitable policies that harness the benefits of nuclear technology while minimising its risks.
Future Prospects and Innovations in Nuclear Chemistry
Nuclear chemistry stands at the forefront of scientific and technological advancements, offering promising prospects and innovations across various fields. From transforming nuclear medicine to revolutionising energy systems and pioneering new research areas, the future of nuclear chemistry is poised to address some of the most critical challenges and opportunities facing society today.
Advancements in Nuclear Medicine
Continuous advancements in nuclear medicine are significantly enhancing diagnostic and therapeutic capabilities. One of the most exciting developments is targeted radionuclide therapy. This technique involves attaching radioactive isotopes to molecules that specifically target diseased cells, such as cancer cells. This method allows for direct radiation delivery to the tumour site, minimising damage to surrounding healthy tissues. For example, Lutetium-177 and Actinium-225 are being investigated for their potential to treat various types of cancers with high precision and efficacy.
Another innovative approach is theranostics, a combination of therapy and diagnostics. This method uses the same radioactive isotopes for both imaging and treatment, providing a personalised medicine approach that can monitor the effectiveness of treatment in real-time and adjust it as needed. Such dual-use isotopes facilitate more accurate diagnosis, better treatment monitoring, and more effective therapy adjustments, significantly improving patient outcomes.
Developments in Nuclear Energy Systems
The future of nuclear energy is focused on making systems safer, more efficient, and more sustainable. Small Modular Reactors (SMRs) are a key development in this field. These reactors are smaller in size and can be built in factories and shipped to sites where they are assembled. SMRs offer several advantages, including reduced initial capital investment, enhanced safety features, and flexibility in location and scaling. They are considered more adaptable to changing electricity markets and can be integrated with renewable energy systems, contributing to a more diverse and stable energy grid.
The push towards Advanced Reactor Designs, such as molten salt reactors, high-temperature gas-cooled reactors, and fast reactors, promises higher efficiencies and the ability to reduce nuclear waste through improved fuel utilisation and transmutation technologies. These innovations aim to address key issues such as safety, waste management, and the non-proliferation of nuclear materials.
Emerging Research Areas
Emerging research areas in nuclear chemistry include the development of new materials and technologies to enhance safety and efficiency in nuclear processes. One area of focus is the improvement of radiation shielding materials through the use of nanotechnology. Nanomaterials can offer better protection against radiation while being lighter and less bulky than traditional materials, which is particularly advantageous for space exploration and medical applications.
Another exciting area is the use of nuclear techniques in environmental protection. Researchers are exploring ways to use isotopes to capture and store carbon or to trace pollutants in ecosystems, helping to mitigate the impacts of climate change and industrial pollution.
In addition, the field of radioisotope power systems is expanding, especially for use in space exploration. These systems, which convert the heat released by the decay of radioactive materials into electricity, are crucial for long-duration missions in space, where solar power is not feasible.
The future of nuclear chemistry is rich with potential, marked by innovations that could redefine medical treatments, transform energy systems, and open new pathways in scientific research. As advancements continue to emerge, they promise significant benefits to public health, energy security, and environmental sustainability, underscoring the critical role of nuclear chemistry in the modern world.
Conclusion
The exploration of nuclear chemistry covers a wide range of critical aspects, from the basic mechanisms of nuclear reactions and the types of radiation to the profound implications of radioisotopes in various fields and the comprehensive safety measures required in handling nuclear materials. The advancements and innovations within this field underscore its significant role in shaping technological progress and addressing key societal challenges.
Summary of Key Points
Nuclear chemistry is integral to many of modern society’s most vital functions. It plays a crucial role in energy production through nuclear reactors, which operate on the principles of nuclear fission and potentially fusion in the future. The application of radioisotopes in medicine has revolutionised diagnostic and therapeutic procedures, providing tools that are pivotal in treating cancers and other diseases. In industry, nuclear techniques are essential for tracing processes, ensuring material integrity, and managing energy systems efficiently.
The field also faces significant challenges, particularly in managing safety and environmental impact of radioactive materials. Regulatory frameworks and international standards are critical in maintaining safety and ensuring the ethical use of nuclear technology, with a continuous need for vigilance and innovation to handle waste and prevent nuclear proliferation.
Challenges and Opportunities Ahead
Nuclear chemistry faces challenges and opportunities that will dictate its trajectory in the coming years. One of the primary challenges is the safe and sustainable management of nuclear waste, an issue that requires innovative solutions to minimise environmental impact and ensure public safety. Additionally, the field must navigate the complexities of nuclear non-proliferation, striving to balance the peaceful uses of nuclear technology with the imperative to prevent its misuse.
Opportunities in nuclear chemistry are abundant and promising, particularly in the development of advanced nuclear energy systems such as SMRs and new reactor technologies that promise higher safety and efficiency. The expansion of nuclear medicine, including advanced imaging techniques and targeted radionuclide therapy, presents another frontier that could vastly improve healthcare outcomes.
Emerging research in applying nuclear science to environmental protection and renewable energy integration also offers substantial benefits. This includes using isotopic techniques for carbon capture and environmental monitoring, which can play a pivotal role in combating climate change and promoting sustainability.
The future of nuclear chemistry is laden with the potential to drive significant progress across multiple domains, from healthcare and energy to environmental preservation. By continuing to advance scientific research, improve regulatory frameworks, and develop new technologies, nuclear chemistry can address its challenges and maximise its contributions to a safer, more sustainable world. The field remains a cornerstone of modern science, with its rich array of applications and its critical role in the global pursuit of innovation and safety.
Disclaimer
The content provided in this article, Exploring Nuclear Chemistry: Innovations, Applications, and Future Prospects, is intended for informational and educational purposes only. While Open MedScience has made reasonable efforts to ensure the accuracy and currency of the information presented, no guarantee is made as to its completeness or reliability.
This article does not constitute professional advice in nuclear science, medicine, engineering, or environmental safety. Readers should consult qualified professionals or regulatory bodies before making decisions based on the topics discussed, particularly those involving health, safety, or technical practices.
References to nuclear technologies, isotopes, or procedures are made in the context of scientific exploration and do not imply endorsement or recommendation for specific uses. Open MedScience accepts no responsibility for any consequences arising from the interpretation or application of the information contained within this publication.
The views expressed are those of the authors and contributors and do not necessarily reflect the official policy or position of Open MedScience or its affiliates.
home » diagnostic medical imaging blog » Features »