This article explores the structure of the atom, from its early conception in ancient Greece to the advanced modern model grounded in quantum mechanics. Concepts such as subatomic particles (protons, neutrons, and electrons), evolution of atomic theory, electron configuration, and isotopes are discussed. Emphasis is placed on the importance of quantum mechanics in explaining electron behaviour and energy levels. The structure of the nucleus and forces acting within it are also examined, including a look at atomic structure, which highlights its fundamental role in the broader understanding of chemistry and physics.
Introduction: The Concept of the Atom
The atom, derived from the Greek word “atomos,” meaning indivisible, is the fundamental unit of matter. Philosophers like Democritus first postulated the existence of indivisible particles around 400 BCE. However, it wasn’t until the 19th and 20th centuries that scientific evidence firmly established the structure of the atom.
Today, the atom is understood as a complex entity, consisting of smaller subatomic particles, governed by laws of quantum mechanics. This shift from classical to quantum theory revolutionised our understanding of matter, leading to advanced technologies and a deeper comprehension of chemical reactions, materials, and the universe itself.
Early Atomic Models: From Indivisible Particles to Electrons
Dalton’s Atomic Theory
In 1808, John Dalton proposed the first modern atomic theory, suggesting that atoms were indivisible and indestructible units that formed the basis of all matter. According to Dalton, each chemical element consisted of identical atoms, and compounds formed when atoms from different elements combined in fixed ratios. Though revolutionary, Dalton’s model did not explain the internal structure of atoms.
J.J. Thomson and the Discovery of the Electron
The discovery of the electron by J.J. Thomson in 1897 significantly altered the atomic model. By conducting experiments with cathode rays, Thomson showed that atoms were not indivisible. Instead, they contained negatively charged particles, which he called electrons. His “plum pudding model” suggested that these electrons were embedded within a positively charged “soup,” creating a balanced structure.
Rutherford’s Nuclear Model
Ernest Rutherford’s famous gold foil experiment in 1909 disproved Thomson’s model. Rutherford demonstrated that atoms had a dense, positively charged nucleus at the centre, where most of the mass was concentrated, while electrons orbited the nucleus. This marked a significant leap towards the modern atomic model. Still, it raised further questions about how electrons remained in stable orbits without spiralling into the nucleus due to electromagnetic attraction.
Subatomic Particles: The Building Blocks of the Atom
Protons
Protons are positively charged subatomic particles found in the nucleus of the atom. Each proton carries a charge of +1 and a mass of approximately 1 atomic mass unit (amu). The number of protons in an atom defines its atomic number and determines the element to which the atom belongs. For instance, hydrogen atoms always have one proton, while carbon atoms have six.
Neutrons
Neutrons are neutral particles, also located in the nucleus. Like protons, they have a mass of about 1 amu, but they carry no electric charge. The number of neutrons in an atom can vary without changing the element, leading to the existence of isotopes, which are atoms of the same element with different numbers of neutrons.
Electrons
Electrons are negatively charged particles with a mass of about 1/1836 of a proton. They are found in regions surrounding the nucleus, forming a cloud that defines the atom’s size and shape. The interaction of electrons between atoms forms the basis of chemical bonding. Unlike protons and neutrons, electrons are not confined to the nucleus, allowing them to participate in various types of chemical reactions.
Quantum Mechanics and Electron Configuration
Bohr’s Model and Quantum Leaps
In 1913, Niels Bohr introduced a model where electrons orbit the nucleus in fixed energy levels or shells. Electrons could “jump” between these levels by absorbing or emitting energy, explaining atomic spectra. Although Bohr’s model was an improvement, it still couldn’t account for the full complexity of electron behaviour, especially in larger atoms.
Quantum Mechanics and the Wave-Particle Duality
The development of quantum mechanics in the 1920s, particularly the work of scientists like Schrödinger and Heisenberg, provided a more complete understanding of the atom. Instead of electrons orbiting in fixed paths, they behave as both particles and waves, a concept known as wave-particle duality.
The Schrödinger equation describes electrons as existing in “orbitals”—regions of space around the nucleus where the probability of finding an electron is highest. These orbitals have different shapes and energy levels, further divided into sublevels known as s, p, d, and f orbitals.
Electron Configuration
Electron configuration refers to the arrangement of electrons in an atom’s orbitals. Each electron occupies the lowest available energy level, filling orbitals in a specific order defined by the Pauli Exclusion Principle, Hund’s Rule, and the Aufbau Principle. Understanding electron configuration is crucial for predicting an atom’s chemical behaviour and bonding characteristics.
For example, the electron configuration of carbon (atomic number 6) is 1s² 2s² 2p², indicating that it has two electrons in its 1s orbital, two in the 2s orbital, and two in the 2p orbital.
The Nucleus: Protons, Neutrons, and Nuclear Forces
Nuclear Structure
The nucleus, comprising protons and neutrons, accounts for nearly all of an atom’s mass. Although protons repel each other due to their positive charges, they are held together in the nucleus by the strong nuclear force, which is significantly stronger than the electromagnetic repulsion at very short distances.
Isotopes and Stability
Atoms of the same element can have different numbers of neutrons, forming isotopes. For example, carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. While the number of protons remains constant in isotopes, the varying number of neutrons can affect the atom’s stability. Unstable isotopes undergo radioactive decay, releasing energy and transforming into other elements over time.
Atomic Mass and Atomic Number
Atomic Number
The atomic number of an element refers to the number of protons in the nucleus of an atom. It defines the element’s identity; for instance, all atoms with six protons are classified as carbon. The atomic number is crucial because it determines an element’s position in the periodic table, as well as its chemical properties.
Mass Number and Atomic Mass
The mass number of an atom is the sum of protons and neutrons in the nucleus. However, the atomic mass, which is often listed on the periodic table, is a weighted average of all naturally occurring isotopes of an element, taking into account their relative abundance.
Atomic Structure and the Periodic Table
Periodic Trends
The periodic table organises elements based on their atomic number and electron configuration, allowing chemists to predict an element’s properties. Trends such as atomic radius, ionisation energy, and electronegativity can be explained by the arrangement of electrons within atoms. As you move across a period from left to right, atoms gain more protons, pulling the electron cloud closer to the nucleus and decreasing the atomic radius.
Chemical Bonding
The way atoms interact with each other depends on their electron configurations. Atoms bond to achieve a stable electron configuration, often following the octet rule, where they seek to have eight electrons in their outermost shell. There are two main types of bonding: covalent and ionic. In covalent bonding, atoms share electrons, while in ionic bonding, one atom donates electrons to another, creating oppositely charged ions that attract each other.
Applications of Atomic Theory
Nuclear Energy
The understanding of atomic structure has paved the way for nuclear energy. When the nuclei of certain isotopes, such as uranium-235, are split (nuclear fission), a tremendous amount of energy is released. This energy is harnessed in nuclear power plants and weapons.
Quantum Chemistry
Quantum mechanics, rooted in atomic theory, plays a central role in understanding chemical reactions and the behaviour of materials at the atomic level. This knowledge is essential for fields such as nanotechnology, semiconductors, and drug design.
Medical Imaging and Treatment
Radioactive isotopes are widely used in medical diagnostics and treatment. For instance, positron emission tomography (PET) relies on radioactive tracers, which emit positrons as they decay, helping to create detailed images of organs and tissues.
Atomic Models in the 21st Century
Advancements in Quantum Mechanics
In the 21st century, our understanding of the atom continues to evolve with advancements in quantum mechanics and particle physics. The behaviour of electrons, protons, and neutrons is now explored in greater detail using high-energy particle accelerators and advanced computational models. Quantum mechanics has expanded to include the study of quantum entanglement and quantum computing, where the behaviour of electrons and other subatomic particles is harnessed for computational power far beyond classical systems.
The Standard Model of Particle Physics
Beyond the traditional view of protons, neutrons, and electrons, the Standard Model of particle physics offers a deeper understanding of the subatomic world. According to this model, protons and neutrons themselves are made up of even smaller particles called quarks, held together by particles known as gluons. Electrons are classified as leptons. The Standard Model also includes force-carrying particles like photons (responsible for electromagnetic forces) and the Higgs boson, which is linked to the concept of mass.
These advancements offer a more detailed understanding of atomic structure, helping to explain phenomena like why certain particles have mass and how forces act at the smallest scales. It marks an ongoing evolution in the study of atomic theory, building on the work of earlier scientists to offer even greater insight into the nature of matter.
Future Directions: Unifying Theories
While the Standard Model has been successful in explaining many aspects of particle physics, scientists continue to search for a “theory of everything” that unifies quantum mechanics with Einstein’s theory of general relativity, which governs the behaviour of gravity on a larger scale. This quest has led to the development of string theory and loop quantum gravity, though these remain theoretical frameworks awaiting experimental validation.
The pursuit of a unified theory is likely to reshape our understanding of the atom and its role in the universe even further. It may also open new doors for technologies that operate on quantum principles, including quantum communication and computing.
Experimental Techniques in Atomic Research
Spectroscopy
One of the key experimental techniques for studying atomic structure is spectroscopy. By analysing the light emitted or absorbed by atoms, scientists can determine the energy levels of electrons and gain insight into the structure of the atom. This technique has been crucial in confirming theoretical models of atomic behaviour.
Spectroscopy also allows for the identification of elements based on their unique atomic spectra, a principle used in both chemistry and astronomy. When elements are heated or exposed to electrical energy, their electrons jump to higher energy levels and then fall back down, emitting light at specific wavelengths. Each element has a unique spectrum, functioning like a fingerprint that can be used to identify it in different contexts.
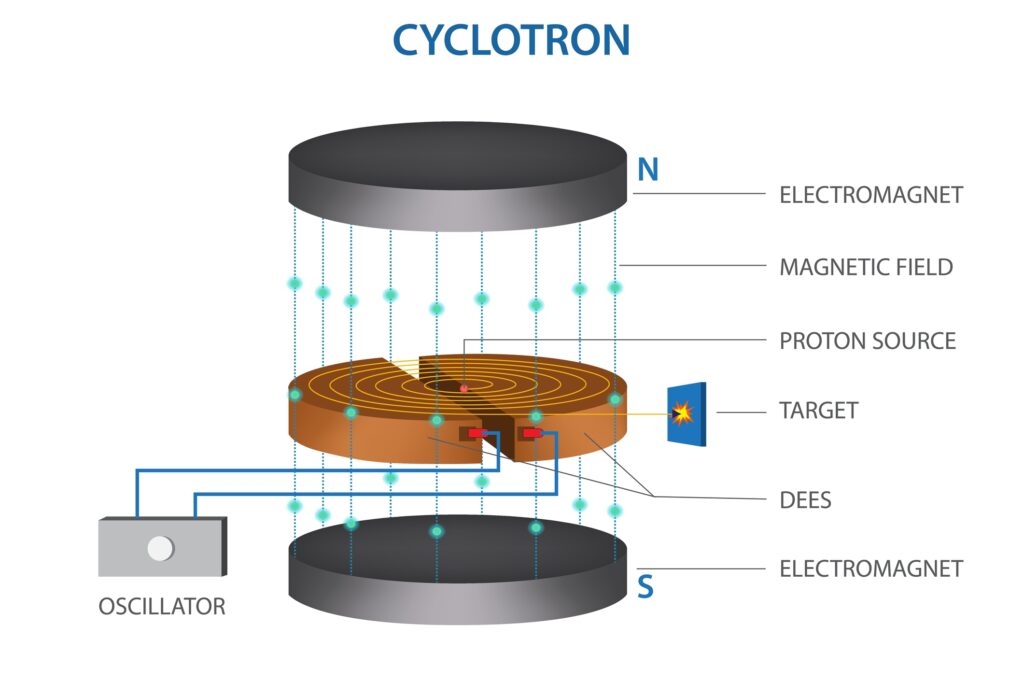
Particle Accelerators
Modern research into atomic structure often involves the use of particle accelerators, such as the Large Hadron Collider (LHC) at CERN. These machines accelerate subatomic particles to incredibly high speeds and smash them together, allowing scientists to study the resulting collisions and the particles produced. This has led to the discovery of new particles, such as the Higgs boson in 2012, and provides valuable data for refining our understanding of atomic structure.
Electron Microscopy
Electron microscopy allows scientists to observe atoms directly, providing visual evidence of atomic structure. Transmission electron microscopes (TEMs) use a beam of electrons to pass through a sample, creating high-resolution images of individual atoms. Scanning tunnelling microscopes (STMs) are even more precise, allowing for the manipulation of individual atoms and the study of atomic-scale properties.
These experimental tools have advanced our understanding of atomic theory significantly and continue to play a vital role in ongoing research.
The Role of Atomic Theory in Modern Science
Chemistry and Material Science
Atomic theory is central to all branches of chemistry. It explains how elements combine to form compounds, the behaviour of gases, and the principles of thermodynamics and reaction kinetics. Understanding the structure of the atom helps chemists design new materials with specific properties, from lightweight alloys to superconductors. In material science, atomic theory is used to create materials at the nanoscale, leading to innovations in electronics, medicine, and engineering.
Biology and Medicine
In biology and medicine, atomic theory underpins our understanding of biochemistry and the molecular mechanisms of life. The structure of atoms influences the behaviour of molecules such as DNA, proteins, and enzymes, all of which are critical for biological processes. In medicine, the principles of atomic structure are applied in technologies like magnetic resonance imaging (MRI), which relies on the behaviour of hydrogen atoms in the body, and radiotherapy, which uses radioactive isotopes to target cancer cells.
Astronomy and Astrophysics
Atomic theory also plays a crucial role in astronomy and astrophysics. By studying the spectra of stars and galaxies, scientists can determine the composition of distant celestial objects and infer their physical properties. Understanding atomic processes helps explain phenomena such as stellar nucleosynthesis, the process by which stars create heavier elements through nuclear fusion. Additionally, atomic theory informs models of the early universe and the formation of matter after the Big Bang.
The Atom as a Gateway to Understanding Matter and the Universe
The atom, once thought to be indivisible, is now understood to be a complex structure composed of subatomic particles governed by the principles of quantum mechanics. The journey from ancient philosophical ideas to modern scientific theory demonstrates how our understanding of the atom has evolved and deepened over time.
The structure of the atom is not only fundamental to the field of chemistry but also forms the basis for our understanding of physics, biology, and even the origins of the universe. As new discoveries in quantum mechanics, particle physics, and experimental techniques continue to emerge, our knowledge of the atom will undoubtedly expand, leading to new technologies and insights into the fabric of reality.
Atomic theory represents both a triumph of human curiosity and a gateway to further exploration. The study of the atom continues to inspire and challenge scientists, driving progress across multiple fields and reshaping our understanding of the universe at both the smallest and largest scales.
Summary of Key Points:
- The concept of the atom has evolved from ancient Greek philosophy to a sophisticated quantum mechanical model.
- Atoms are made up of three main subatomic particles: protons, neutrons, and electrons, each with distinct properties.
- Quantum mechanics provides a detailed understanding of electron behaviour, replacing older classical models.
- The nucleus contains protons and neutrons, held together by the strong nuclear force, while electrons occupy regions around the nucleus known as orbitals.
- Isotopes are atoms with the same number of protons but different numbers of neutrons, leading to variations in atomic mass.
- The periodic table organises elements based on their atomic structure and helps predict chemical behaviours.
- The discovery of quarks, gluons, and other fundamental particles has deepened our understanding of atomic structure through the Standard Model of particle physics.
- Experimental techniques such as spectroscopy, particle accelerators, and electron microscopy are crucial for studying atoms.
- Atomic theory has wide-ranging applications, from material science and chemistry to biology, medicine, and astrophysics.
Disclaimer
This article is intended for informational and educational purposes only. While every effort has been made to ensure the accuracy and reliability of the content, Open Medscience does not guarantee its completeness or scientific precision. The material presented reflects current understanding as of the date of publication and may not include the most recent developments in atomic theory or quantum mechanics.
The information provided should not be used as a substitute for professional advice or consultation with qualified experts in the fields of chemistry, physics, or related sciences. Open Medscience shall not be held responsible for any errors, omissions, or consequences arising from the use or interpretation of the material contained herein.
Readers are encouraged to consult primary scientific literature and educational institutions for more detailed or updated information.
You are here: home » diagnostic medical imaging blog »