Brachytherapy techniques have been a powerhouse in the treatment of cancer since the beginning of the twentieth century.
Types of Brachytherapy
Brachytherapy techniques have been a powerhouse in the treatment of cancer since the beginning of the twentieth century. The advancement of other theranostic treatments has not removed internal radiotherapy entirely from the clinician’s armoury war in the ongoing quest to destroy cancer.
One century forward, brachytherapy, although in some cases, not always an effective radiotherapy treatment, continues to play a fundamental role in cancer therapy. Brachytherapy treatment for prostate cancer, gynaecological, skin and breast cancer treatments is believed to offer continuing treatment possibilities through technological developments in the future.
Brachytherapy means merely short therapy. It is used in radiotherapy by applying a sealed radiation source inside the human body. The radiation source is usually placed near where the treatment is required. This type of therapy aims to damage the DNA of cancer cells and ultimately kill them. In the treatment of prostate cancer, brachytherapy can be used in the following modes: low dose rate and high dose rate.
A low dose rate uses a smaller-strength radioactive source and is associated with longer treatment times, especially for one-off treatments. The primary use of low-dose-rate treatment is the prostate, which involves inserting tiny radioactive seeds inside the prostate tissue. This low-dose rate approach has been used in the treatment of head and neck tumours, during which low-activity sealed sources are temporarily placed at the cancer source for several days and then removed.
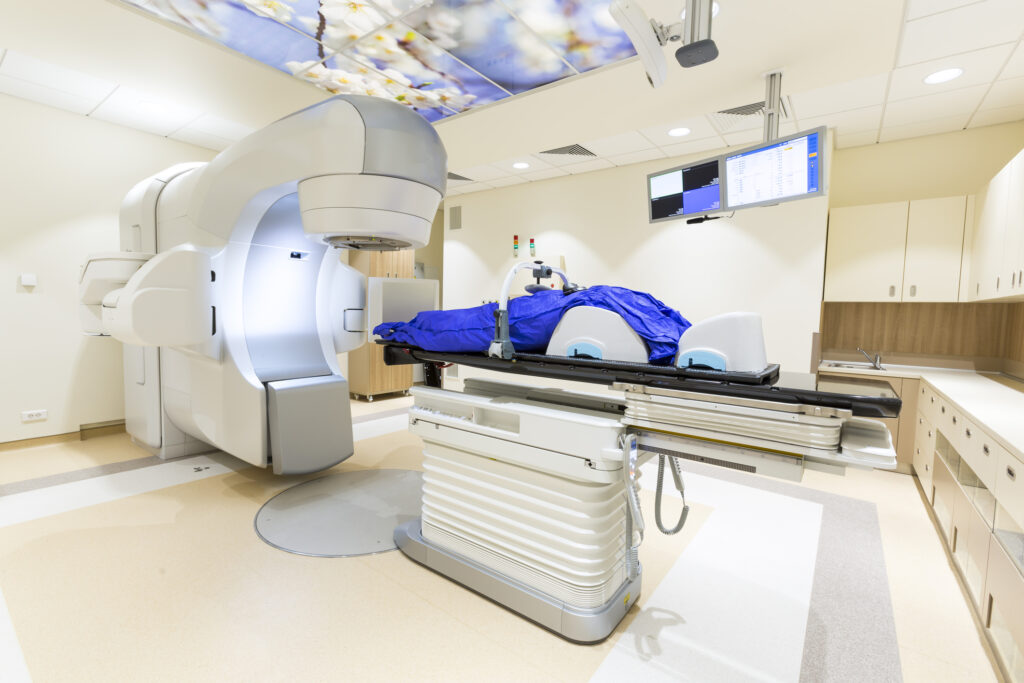
High dose rate applies to a higher strength of radioactive source contained within the after-loader device. The aim of the after-loader is to deliver the radioactive source in the vicinity of the tumour for a brief period of time. This is usually facilitated by the use of catheters and needles which are inserted into the tumour site.
High-dose-rate treatment is a much shorter procedure—minutes compared to several days of low-dose-rate treatments. However, it requires multiple sessions and has become the more favourable option to replace low-dose-rate techniques for most body sites.
In addition, other internal radiotherapy radiation techniques include pulse dose rate and image-guided brachytherapy.
Pulsed dose rate brachytherapy treatment combines the physical advantages of high-dose-rate technology with the radiobiological advantages of low-dose-rate brachytherapy. Pulsed brachytherapy consists of using a stronger radiation source than low-dose-rate brachytherapy and produces a series of short exposures of up to 30 minutes every hour to approximately the same total dose in the same overall time as with low-dose-rate brachytherapy.
Advancements in afterloading equipment provide several advantages over the intracavitary insertion of separate tubes, wires, needles, and seeds. An automated approach removes the internal radioactive source from the patient to reduce radiation exposure. The radiation exposure is also reduced for the staff who formerly loaded and unloaded a multiplicity of radioactive sources into the catheters and tubes.
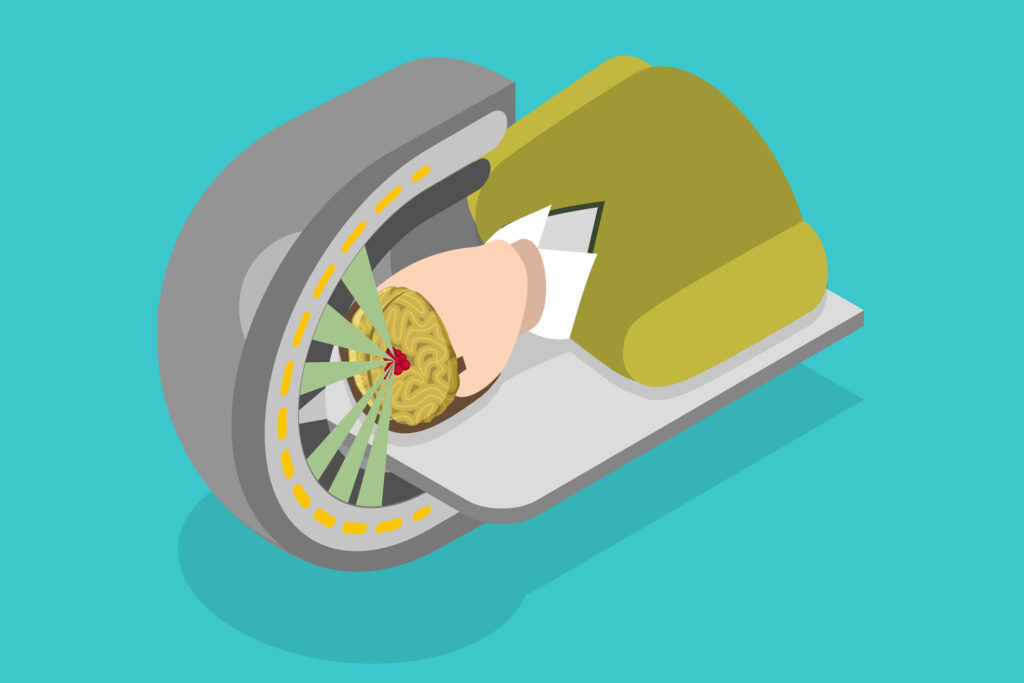
Image-guided brachytherapy involves using advanced imaging techniques to make brachytherapy more accurate, safe, and effective. Internal radiotherapy consists of four phases: placement of hollow catheters or hollow carriers; computed tomography or magnetic resonance imaging of the site; computer calculations of the dose distribution (dosimetry); and robotic radiation treatment delivery with a tiny radiation source. The imaging techniques used for interventional radiology may be used to guide the placement of the internal radiotherapy catheters.
Emerging Techniques and Future Directions
As the field of internal therapy continues to evolve, several emerging techniques and technological advancements promise to enhance its efficacy and safety further. These innovations are paving the way for more precise, targeted, and patient-friendly cancer treatments.
Intraoperative Brachytherapy (IOBT)
Intraoperative brachytherapy (IOBT) is a technique in which radiation is applied during surgery. This allows for direct visualisation of the tumour site and precise placement of the radiation source. IOBT is particularly useful in treating cancers that are difficult to reach or where residual microscopic disease is suspected. By delivering radiation during surgery, IOBT minimises radiation exposure to surrounding healthy tissues and allows for higher doses to be delivered directly to the tumour.
Real-Time Brachytherapy
Real-time brachytherapy involves the use of advanced imaging and computer technology to guide the placement of radioactive sources in real-time. This technique improves source placement accuracy and allows adjustments to be made during the procedure. Real-time brachytherapy is particularly beneficial for treating complex tumours and those in sensitive areas where precision is paramount.
Electronic Brachytherapy (eBx)
Electronic brachytherapy uses a miniaturised X-ray source instead of radioactive isotopes. This technology eliminates the need for radioactive materials, thereby reducing regulatory and safety concerns associated with handling and disposal. Electronic brachytherapy can be used for skin, breast, and other malignancies. It offers the advantage of being a portable and convenient option for delivering high-dose radiation therapy in an outpatient setting.
Surface Brachytherapy
Surface brachytherapy, or plesiotherapy, is used for treating superficial cancers such as skin cancer. It involves placing a radiation source close to the skin surface, allowing for a concentrated dose of the tumour while sparing deeper tissues. Advances in applicator design and imaging techniques have improved the precision and effectiveness of surface brachytherapy, making it a valuable option for treating non-melanoma skin cancers and other superficial lesions.
Brachytherapy for Non-Malignant Conditions
While internal therapy is predominantly used for cancer treatment, its applications are expanding to include non-malignant conditions. For example, after angioplasty, brachytherapy is being explored as a treatment for benign tumours, keloids, and vascular restenosis. The ability to deliver localised radiation with minimal side effects makes brachytherapy an attractive option for these conditions.
Radiopharmaceuticals and Brachytherapy
The integration of radiopharmaceuticals with brachytherapy is an area of active research. Radiopharmaceuticals are radioactive compounds that can target specific tissues or cellular receptors. When combined with brachytherapy, these agents can enhance the therapeutic effect by delivering radiation directly to cancer cells while minimising exposure to healthy tissues. This combination approach has the potential to improve outcomes for patients with metastatic or recurrent cancers.
Patient-centred approaches and Personalised Brachytherapy
Personalised medicine is making its way into brachytherapy, with treatment plans increasingly tailored to the individual characteristics of the patient and their tumour. Advanced imaging techniques, genetic profiling, and artificial intelligence are being used to customise brachytherapy dosages and delivery methods. These personalised approaches aim to maximise treatment efficacy while minimising side effects and improving the overall patient experience.
Conclusion
The future of brachytherapy looks promising with continuous advancements in technology and treatment methodologies. Emerging techniques such as intraoperative, real-time, and electronic brachytherapy enhance this treatment modality’s precision and effectiveness. Additionally, the expanding applications of brachytherapy to non-malignant conditions and the integration of personalised approaches underscore its versatility and potential in modern cancer care. As these innovations continue to develop, brachytherapy is poised to remain a critical component of comprehensive cancer treatment strategies, offering hope and improved outcomes for patients worldwide.
Disclaimer
The information presented in this article is for general educational and informational purposes only. It is not intended as a substitute for professional medical advice, diagnosis, or treatment. Brachytherapy and other internal radiotherapy techniques should only be considered and administered under the guidance of qualified healthcare professionals.
Open Medscience does not endorse or promote any specific medical treatment, device, or pharmaceutical product mentioned in this article. While efforts are made to ensure the accuracy and relevance of the content, medical knowledge and practices are constantly evolving, and readers are encouraged to consult appropriate medical professionals before making decisions about their healthcare.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of Open Medscience or its affiliates.
You are here: home » diagnostic medical imaging blog »