Nuclear medicine has evolved over a century, profoundly enhancing diagnostics and treatments in healthcare.
Early Discoveries and Radioactive Elements (1896-1930)
Nuclear medicine is a fascinating and evolving field that intertwines advanced technology, chemistry, physics, and medicine to diagnose and treat diseases. Its history spans over a century, beginning with the discovery of radioactivity and the development of radionuclides for medical use.
The history of nuclear medicine is deeply rooted in the foundational discoveries of the late 19th and early 20th centuries. Key figures like Henri Becquerel and Marie Curie played pivotal roles in unveiling the properties of radioactive elements, laying the groundwork for the medical application of these discoveries.
Discovery of Radioactivity (1896)
- Henri Becquerel’s Discovery: Henri Becquerel’s discovery of natural radioactivity in 1896 was a landmark moment. Investigating phosphorescence in uranium salts, he unexpectedly found that these salts emitted rays that could penetrate solid objects and fog photographic plates, similar to the recently discovered X-rays by Wilhelm Conrad Roentgen. This revelation of natural radioactive emissions set the stage for a new field of scientific inquiry.
- Impact on Medicine: Becquerel’s discovery was not immediately linked to medicine. However, it opened a path to understanding atomic structure and radioactivity, which would later prove crucial for diagnostic and therapeutic applications in medicine.
Marie Curie’s Contributions
- Discoveries of Radium and Polonium: Marie Curie, along with her husband Pierre, extended Becquerel’s work, discovering new radioactive elements – radium and polonium. These discoveries were monumental, as Curie isolated and characterised these elements, demonstrating their unique properties.
- Foundations for Medical Application: Marie Curie’s pioneering research helped establish the fundamental principles of radioactivity. Her work led to the realisation that radioactive substances could be used to study and treat diseases, especially with the notable intensity and energy of the radiation emitted by radium.
First Medical Use of Radioactive Isotopes (1913)
- Georg de Hevesy’s Experiment: The first recorded use of radionuclides in medicine was in 1913 by Georg de Hevesy. He used lead-212, a radioactive isotope, to investigate the absorption and translocation of lead in plant systems.
- Significance for Nuclear Medicine: While Hevesy’s experiment was primarily botanical, it demonstrated the potential of using radioactive isotopes as tracers. This concept would become central to nuclear medicine, allowing doctors to track the movement and concentration of substances within the body.
The Broader Impact
These early discoveries and applications of radioactive elements and isotopes were crucial stepping stones. They advanced scientific understanding of atomic physics and paved the way for the development of nuclear medicine. The ability to use radioactive isotopes as tracers or therapeutic agents revolutionised diagnostic imaging and treatment, leading to groundbreaking techniques like PET scans, SPECT, and radiotherapy for cancer treatment.
The work of Henri Becquerel and Marie Curie, in particular, exemplifies the profound impact that fundamental scientific research can have on practical applications, leading to advancements that continue to benefit humanity in numerous ways.
Technological Advancements and Therapeutic Applications (1930-1950)
The 1930s and 1940s were pivotal decades in the evolution of nuclear medicine, marked by significant technological advancements and practical applications. These years saw the development of critical tools and techniques that expanded the potential of nuclear medicine in both diagnostics and treatment.
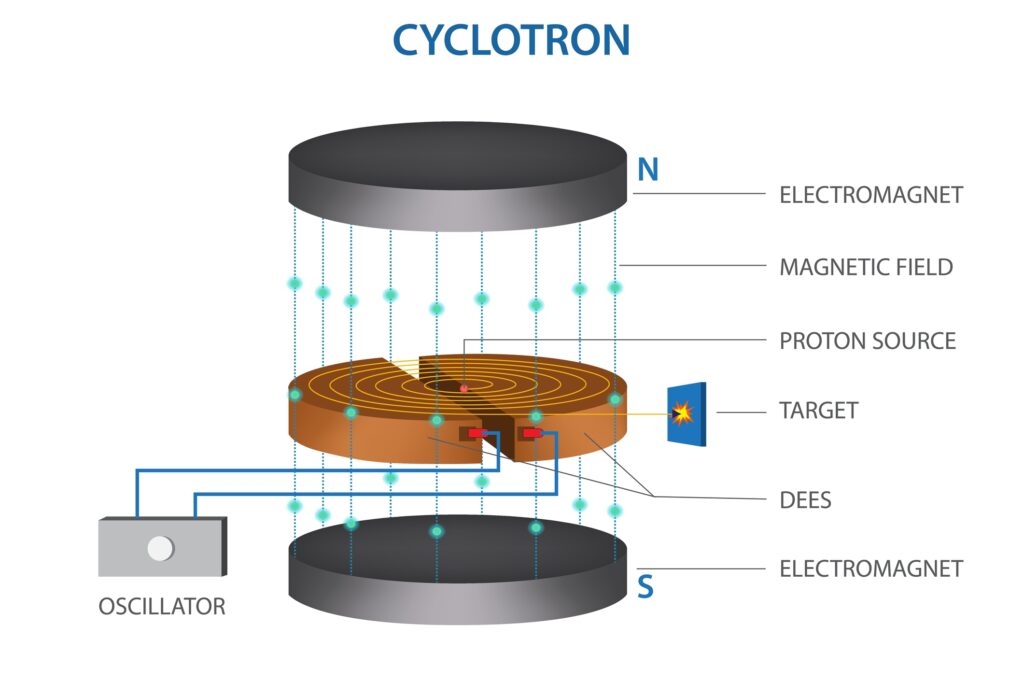
Development of Cyclotrons (1930s)
- Ernest O. Lawrence’s Invention: The cyclotron, invented by physicist Ernest O. Lawrence in the 1930s, was groundbreaking. This device accelerated charged particles to high energies, enabling the bombardment of materials to produce artificial radionuclides.
- Impact on Radionuclide Production: Before the cyclotron, researchers relied on naturally occurring radionuclides, which were scarce and limited in variety. The cyclotron created a broader range of radionuclides, which were essential for expanding the capabilities of nuclear medicine, especially in diagnostics.
Radioiodine in Thyroid Studies (1930s)
- Saul Hertz’s Research: In the late 1930s, Dr. Saul Hertz, along with his colleagues at Massachusetts General Hospital, conducted pioneering research using radioactive iodine. They explored its uptake in the thyroid gland, providing new insights into thyroid physiology.
- Therapeutic Application: This research led to the first therapeutic use of nuclear medicine. By the early 1940s, radioactive iodine was being used to treat hyperthyroidism and, later, thyroid cancer. This represented a major milestone in the application of radionuclides for disease treatment.
World War II and the Manhattan Project (1940s)
- Advancements in Nuclear Technology: The development of nuclear technology during World War II, particularly through the Manhattan Project, dramatically accelerated the understanding and capabilities surrounding nuclear reactions and radionuclide production.
- Post-War Benefits to Medicine: While the primary goal of these efforts was the development of nuclear weapons, the advancements had significant peacetime applications. Post-war, the technology and knowledge gained were redirected to peaceful uses, including medical research. This led to an increased availability of radionuclides and further innovation in nuclear medicine techniques.
Broader Significance
These developments in the mid-20th century were critical in transforming nuclear medicine from a theoretical concept to a practical tool with real clinical applications. The invention of the cyclotron opened up new possibilities in radionuclide production, while the use of radioiodine in thyroid studies established the role of nuclear medicine in treatment. Finally, the technological advancements driven by World War II efforts significantly enhanced the capabilities of nuclear medicine, laying the groundwork for its expansion in the subsequent decades.
Together, these advancements underscore how scientific progress, often driven by diverse motivations and contexts, can converge to create substantial improvements in medical diagnosis and treatment, ultimately benefiting patient care worldwide.
Growth of Diagnostic Techniques and Therapies (1950-1970)
The 1950s and 1960s marked a transformative era in nuclear medicine, characterised by significant advancements in diagnostic imaging technologies and the introduction of new radionuclides that enhanced imaging capabilities.
First Diagnostic Scans (1950s)
- Emergence of Radionuclide Imaging: In the 1950s, the first diagnostic scans using radionuclides were performed, marking a pivotal moment in medical imaging. These scans utilised radioactive isotopes to visualise internal organs and structures.
- Brain and Liver Scans: Early applications included brain and liver scans. These scans provided unprecedented insights into the functioning of these organs, allowing for the visualisation of abnormalities and diseases that were previously difficult to diagnose non-invasively.
Invention of the Gamma Camera (1957)
- Hal Anger’s Innovation: The gamma camera, developed by Hal Anger in 1957, was a revolutionary invention in the field of nuclear medicine. Unlike earlier detectors, the gamma camera could produce detailed images of the distribution of radioactivity in the body.
- Impact on Nuclear Imaging: This technology enabled more precise and comprehensive imaging, facilitating the diagnosis of various conditions. Its ability to create images from gamma rays emitted by radionuclides within the body opened new possibilities in diagnostics, from cancer detection to cardiac imaging.
Technetium-99m (1960s)
- Introduction of Technetium-99m: The 1960s witnessed the introduction of Technetium-99m (Tc-99m) in nuclear medicine. Tc-99m quickly became a cornerstone in diagnostic imaging due to its ideal physical and chemical properties.
- Advantages and Applications: Tc-99m has a short half-life, emits gamma rays suitable for imaging, and can be incorporated into various compounds to target different organs and systems. This versatility made it highly valuable for a wide range of diagnostic procedures, including bone scans, cardiac stress tests, and cancer detection.
Broader Implications
These developments fundamentally changed the landscape of medical imaging and diagnosis. The ability to conduct non-invasive internal scans opened new avenues in medical diagnosis, allowing for earlier detection and better management of diseases. The gamma camera’s ability to provide detailed, dynamic images revolutionised the way physicians could visualise and understand physiological processes and abnormalities in the body.
The introduction of Technetium-99m further expanded the capabilities of nuclear medicine, providing a versatile and efficient means to explore and diagnose a variety of health issues. Its widespread adoption underscored the growing importance of nuclear medicine in routine clinical practice.
Overall, these advancements in the mid-20th century significantly enhanced nuclear medicine’s diagnostic accuracy and scope, contributing to its establishment as an indispensable tool in modern healthcare.
Expansion and Diversification (1970-Present)
The latter part of the 20th and early 21st centuries have been marked by remarkable advancements in nuclear medicine, notably in imaging technologies and therapeutic applications. These developments have significantly enhanced the ability to diagnose and treat various medical conditions, especially cancer.
PET and SPECT Imaging (1970s-1980s)
- Development of PET and SPECT: In the 1970s and 1980s, two major imaging technologies emerged in nuclear medicine: Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT).
- Functional Imaging Capabilities: Both PET and SPECT represented a significant leap forward because they offered more than just structural imaging; they provided functional and metabolic information. PET scans are particularly effective in detecting metabolic activity in tissues, making them highly valuable in oncology, neurology, and cardiology. While offering slightly lower resolution than PET, SPECT is more widely available and less expensive, making it a valuable tool for various clinical applications.
Therapeutic Advances (1990s-2000s)
- Radioimmunotherapy and Targeted Therapies: The 1990s and 2000s saw significant advancements in the therapeutic applications of nuclear medicine. This period witnessed the rise of radioimmunotherapy and targeted radionuclide therapies, which allowed for more precise targeting of cancer cells while minimising damage to healthy tissues.
- Improved Cancer Treatment: These approaches involve attaching radioactive particles to antibodies or other molecules that specifically target cancer cells. This method has proven particularly effective in treating certain types of cancers, such as lymphoma and prostate cancer, providing a more personalised and effective treatment option.
Pioneering Work of Krenning’s Team in PRRT and the Commercial Success of TheraSphere
Eric Krenning and his team, including Dick Kwekkeboom and Marion de Jong, made significant contributions to the field of nuclear medicine in the late 1990s, particularly in pioneering Peptide Receptor Radionuclide Therapy (PRRT). This innovative approach involves targeting specific receptors on tumour cells with radiolabeled peptides, offering a new avenue for treating certain types of cancers, such as neuroendocrine tumours.
Additionally, there has been considerable work on radiolabeled antibodies using isotopes like Iodine-131 (I-131) and Yttrium-90 (Y-90), which led to regulatory approval. Although these therapies might not have achieved commercial success, they represent necessary steps in the development of targeted cancer therapies.
TheraSphere, involving Y-90 microspheres, is another noteworthy advancement in this field. Developed by scientists including G. Ehrhardt at the Missouri University Research Reactor (MURR), TheraSphere is used for radioembolisation, a process where radioactive beads are delivered directly to liver tumours. This technique has proven to be both innovative and commercially successful, offering a minimally invasive treatment option for patients with certain types of liver cancer.
These developments underscore nuclear medicine’s continuous evolution and impact in cancer therapy, blending innovative scientific research with practical, clinically effective solutions.
The Pioneering Work of Maurits Geerlings and Leaders in Radioligand Therapies
Maurits Geerlings and other pioneers in Radioligand Therapies, like Richard Baum, Rod Hicks, Mike Sathekge, and others, have made monumental contributions to the field of theranostics, a blend of therapy and diagnostics. Their work, particularly in the development and application of actinium-225 (Ac-225), has paved the way for significant breakthroughs in the treatment of various diseases, notably cancer.
Maurits Geerlings and the Advancement of Ac-225
Maurits Geerlings has been a notable figure in the field of nuclear medicine, particularly in the advancement of Ac-225-based therapies. Ac-225, an alpha-emitting radionuclide, has shown great potential in targeted alpha therapy (TAT), a form of radioligand therapy (RLT). This approach involves using molecules that specifically target cancer cells, labelled with a radioactive substance like Ac-225, to deliver a highly localised dose of radiation that kills cancer cells while minimising damage to healthy tissue.
Geerlings’ work has been instrumental in optimising the production and application of Ac-225. He has focused on ensuring a stable supply of this isotope, which is crucial for widespread clinical use. His efforts have involved developing innovative methods for Ac-225 production, including the use of linear accelerators and nuclear reactors.
Richard Baum and the Early Days of Theranostics
Dr. Richard Baum was a pioneer in theranostics, a term he helped popularise. His work in the early 2000s, particularly with Lutetium-177 (Lu-177) based therapies, laid the groundwork for later advancements with Ac-225. Baum’s research focused on using radiolabeled peptides to target specific receptors expressed by tumour cells, such as the somatostatin receptor in neuroendocrine tumours. This approach allowed for both imaging and treatment, hence the term theranostics.
Baum’s work demonstrated the potential of targeted radionuclide therapy to be both effective and have fewer side effects compared to conventional therapies, paving the way for further exploration and development in the field.
Rod Hicks and the Integration of Diagnostic Imaging
Professor Rod Hicks, from Australia, has been instrumental in integrating diagnostic imaging with radionuclide therapy. His work has emphasised the importance of positron emission tomography (PET) and computed tomography (CT) in planning and monitoring radionuclide therapy. Hicks’ contributions have been vital in the development of personalised medicine approaches in oncology, where treatments are tailored based on the specific characteristics of a patient’s tumour, as revealed by advanced imaging techniques.
Mike Sathekge and the Expansion in Clinical Applications
Dr. Mike Sathekge, from South Africa, has significantly contributed to expanding the clinical applications of radioligand therapies in the African context. His work has focused not only on cancers traditionally treated with RLT, like prostate and neuroendocrine tumours but also on exploring the potential for treating other types of cancers prevalent in Africa.
Sathekge’s research has been crucial in demonstrating the effectiveness of RLT in a diverse range of patient populations and has played a key role in making these treatments more accessible in regions where they were previously unavailable.
The Current Landscape and Future Prospects
The collective work of these pioneers has triggered an explosion of interest and development in the field of radioligand therapy and theranostics. We are currently witnessing an expansion in the types of cancers being treated with these methods, along with ongoing research into new radioligands and targets.
One of the key challenges remains the production and supply of radionuclides like Ac-225. Efforts by Geerlings and others in this area are crucial to the continued growth and application of these therapies.
The groundbreaking initiatives and work of Maurits Geerlings and his peers have been fundamental in the evolution of radioligand therapies. Their pioneering efforts have opened up new avenues for cancer treatment, leading to more effective and personalised therapies with fewer side effects. As research continues, the potential for these therapies to treat a broader range of diseases looks increasingly promising, indicating a bright future for the field of theranostics.
Integration with Other Imaging Modalities (2000s-Present)
- Hybrid Imaging Technologies: More recently, the integration of nuclear medicine techniques with other imaging modalities like MRI (Magnetic Resonance Imaging) and CT (Computed Tomography) has led to the development of hybrid imaging technologies.
- Enhanced Diagnostic and Treatment Planning: Combining the functional imaging of PET or SPECT with the anatomical detail provided by MRI or CT has greatly improved diagnostic accuracy and treatment planning. This integration allows clinicians to simultaneously evaluate the structure and function of tissues and organs, leading to more precise diagnoses and personalised treatment strategies.
Broader Impact
These advancements have profoundly impacted the field of nuclear medicine and healthcare as a whole:
- Improved Patient Outcomes: The ability to accurately diagnose and effectively treat various diseases, especially cancer, has improved patient outcomes and quality of life.
- Personalised Medicine: The advancements in targeted therapies and hybrid imaging have paved the way for more personalised medicine, where treatments are tailored to the individual characteristics of each patient’s disease.
- Research and Innovation: The ongoing development in imaging and therapeutic technologies continues to drive research and innovation in nuclear medicine, promising even more sophisticated and effective tools in the future.
Significant technological and therapeutic advancements have characterised the evolution of nuclear medicine over the past few decades. These developments have enhanced our understanding of numerous diseases and provided powerful tools for diagnosis and treatment, contributing to the broader field of personalised medicine.
Future Directions
The future of nuclear medicine is being shaped by several exciting trends and advancements, particularly in the area of personalised medicine, advanced radiopharmaceuticals, and the integration of nanotechnology. These developments are poised to significantly enhance the precision and effectiveness of nuclear medicine in both diagnostics and therapy.
Personalised Medicine
- Tailored Treatments: The concept of personalised medicine in nuclear medicine involves tailoring diagnostic procedures and therapies to the individual’s specific biological characteristics. This approach aims to optimise the effectiveness of treatment by considering factors like genetic makeup, molecular/cellular analysis, and individual health history.
- Impact on Patient Care: Personalised nuclear medicine can lead to more effective treatment with fewer side effects, as therapies are specifically designed to target the patient’s unique disease process. This approach is particularly promising in oncology, where the exact type and location of cancer cells can significantly influence treatment strategy.
Advanced Radiopharmaceuticals
- Development of New Agents: Ongoing research in nuclear medicine is focused on developing new and more effective radiopharmaceuticals. These are drugs that contain radioactive substances and are used to diagnose or treat various diseases.
- Improving Diagnosis and Treatment: The goal of these advancements is to improve the specificity and efficacy of diagnostic imaging and therapeutic procedures. New radiopharmaceuticals could offer better localisation of diseases, more precise imaging, and targeted therapy with minimal impact on healthy tissues.
Integration with Nanotechnology
- Nanotechnology in Nuclear Medicine: The integration of nanotechnology into nuclear medicine is an area of significant potential. Nanotechnology involves working with materials at the atomic or molecular level, which can be applied to design more effective radiopharmaceuticals and imaging agents.
- Enhanced Disease Targeting: By leveraging nanotechnology, researchers aim to create agents that can target diseases at a cellular or even molecular level. This precise targeting could lead to earlier detection of diseases and more effective treatments, especially in the case of cancer, where early detection and targeted therapy are crucial.
Broader Implications
- Revolutionising Healthcare: These advancements are expected to revolutionise the field of nuclear medicine and have a broader impact on healthcare. They promise to improve the accuracy of diagnoses and the effectiveness of treatments and ultimately lead to better patient outcomes.
- Research and Ethical Considerations: As these technologies develop, they will also raise important questions about cost, accessibility, and ethical considerations, including how best to use these powerful tools responsibly.
The future of nuclear medicine is bright and dynamic, with personalised medicine, advanced radiopharmaceuticals, and the integration of nanotechnology leading the way. These advancements hold the promise of transforming the way diseases are diagnosed and treated, offering hope for more effective, efficient, and personalised healthcare solutions.
Conclusion
The journey of nuclear medicine from its inception to its current state is a remarkable story of scientific ingenuity and medical progress. This field has consistently pushed the boundaries of what’s possible in healthcare, transforming the way we understand, diagnose, and treat a myriad of diseases.
- From Discovery to Innovation: Beginning with the foundational discovery of radioactivity, nuclear medicine has evolved through groundbreaking innovations like the development of the cyclotron, the advent of radionuclide imaging techniques, and the creation of sophisticated imaging technologies such as PET and SPECT. Each of these milestones has contributed significantly to the field’s evolution.
- Improving Diagnosis and Treatment: Nuclear medicine has revolutionised the approach to diagnosing and treating various conditions, particularly cancer. Techniques like radioimmunotherapy and the use of advanced radiopharmaceuticals have opened new horizons in targeted therapy, significantly improving patient outcomes.
- Impact on Patient Care: The advancements in nuclear medicine have not only provided more effective diagnostic and treatment options but have also enhanced the precision of medical interventions, leading to more personalised and effective patient care. This precision has been further enhanced by the integration of nuclear medicine with other imaging modalities and the promising incorporation of nanotechnology.
- Looking to the Future: As we look forward, the potential for further innovation in nuclear medicine is immense. The ongoing research in personalised medicine, advanced radiopharmaceuticals, and the integration of emerging technologies like nanotechnology points to a future where nuclear medicine will continue to play a pivotal role in advancing healthcare.
- A Testament to Human Endeavour: The history of nuclear medicine is a testament to the relentless human endeavour to understand and utilise the natural world for the betterment of health. It demonstrates how interdisciplinary collaboration across physics, chemistry, biology, and medicine can yield extraordinary benefits to society.
Nuclear medicine stands as a prime example of how scientific discovery and technological innovation can profoundly impact healthcare. As the field continues to evolve, it holds the promise of even more remarkable achievements in the diagnosis and treatment of diseases, further improving the quality of life for patients around the globe.
Disclaimer
The content presented in “History of Nuclear Medicine: A Century of Innovation and Impact” is intended for informational and educational purposes only. While every effort has been made to ensure the accuracy of the historical and scientific information, Open Medscience does not guarantee the completeness or timeliness of the material. The article does not constitute medical advice, professional consultation, or endorsement of any specific treatment, technology, or institution mentioned.
Readers are encouraged to consult qualified healthcare professionals for advice and guidance on any medical condition or treatment. References to historical figures, research findings, and developments are based on publicly available information and are not intended to imply direct association with or endorsement by the individuals or organisations mentioned.
Open Medscience disclaims any liability for actions taken based on the content of this article.
home » diagnostic medical imaging blog » Open Med | Features »