Alzheimer’s plaque imaging
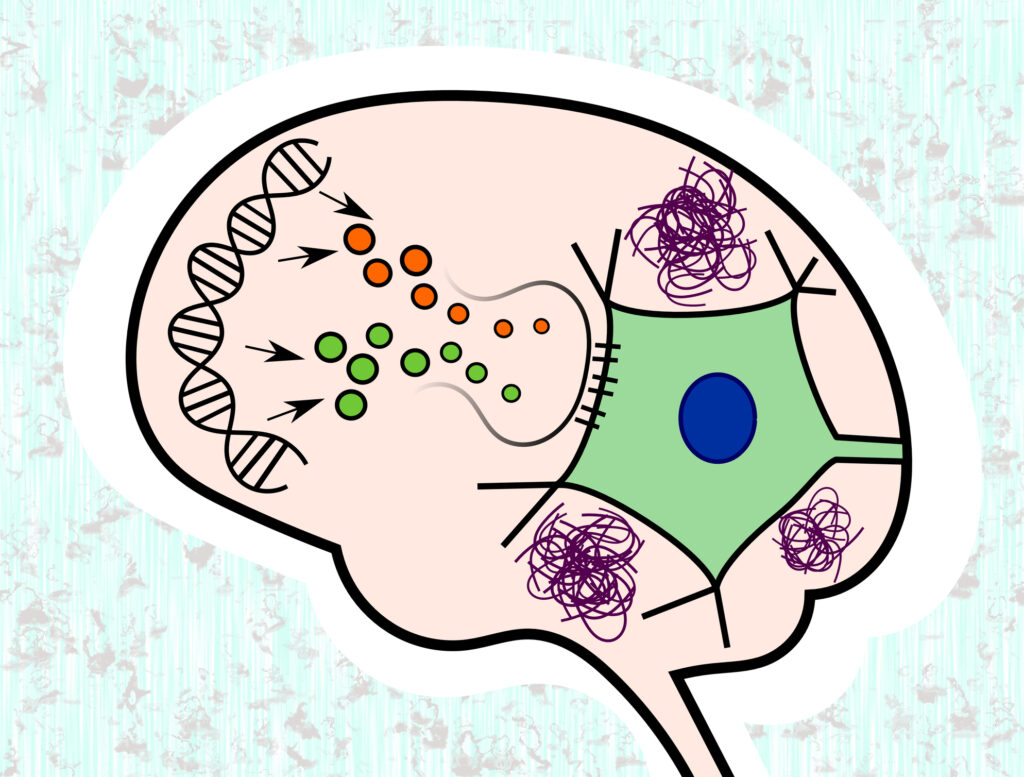
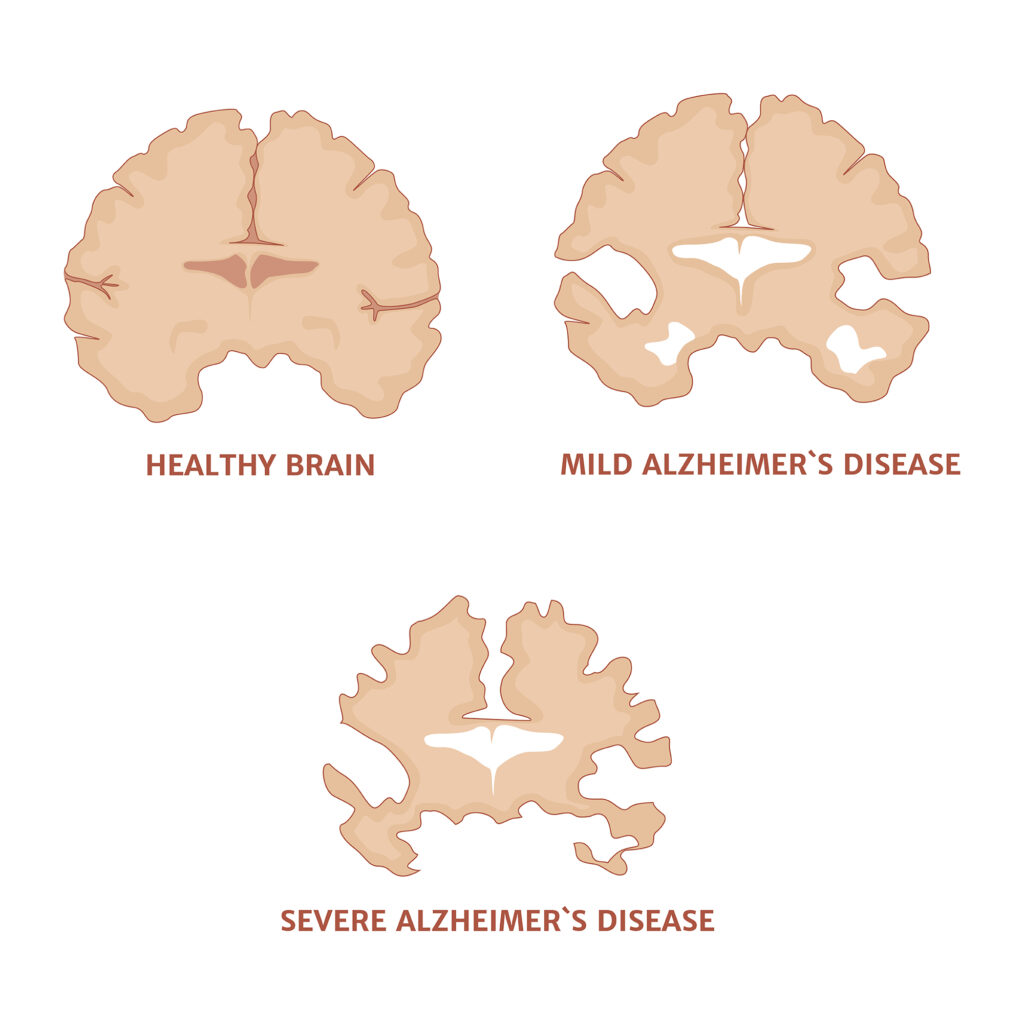
Alzheimer’s plaque imaging is used to evaluate Alzheimer’s disease, caused by the build-up of amyloid plaques in the brain. Alzheimer’s disease is a neurodegenerative disorder with clinical appearances of progressive memory decline associated with loss of language. This deliberating disease affects about 5.3 million Americans, mostly over the age of 65. Alzheimer’s disease is characterised by aggregated amyloid-beta (Aβ) accumulation in the brain. These are the pathological features of Alzheimer’s disease (AD)-related amyloid β-peptide (Aβ) pathology plaques.
The amyloid-beta (Aβ) consists of a 38-43 amino acid peptide derived from the proteolytic cleavage of the amyloid precursor protein. These Aβ plaques have been investigated using advanced brain imaging and quantitative techniques to measure the level of Aβ in biological fluids. However, there is a strong association between amyloid plaques with AD dementia. It has been shown that amyloid deposition occurs over at least 20 years before the onset of dementia. Also, it has been shown that the production and clearance of Aβ in cerebrospinal fluid demonstrates the clearance of Aβ42 is decreased in Alzheimer’s disease.
Therefore, PET imaging has shown that amyloid plaque increases slowly, correlating with the clinical presentation of dementia. However, animal model studies of plaques have indicated that plaques grow to a stable size and may be in equilibrium with their environment. PET imaging of the human brain gives an average measure of amyloid pathology over 1 cm.
However, PET imaging does not measure individual amyloid plaques; the amyloid tracer binds to a subset of aggregated Aβ. Therefore, PET imaging is limited due to amyloid binding agents measuring the binding sites, resulting in a lower amount of amyloid plaques. The stable isotope labelling kinetics (SILK) technique was developed to allow the quantitation of human protein turnover.
This approach involved nanoscale secondary ion mass spectrometry (NanoSIMS), which facilitates both the imaging and measurement of stable isotopes (13C6-leucine) at high spatial resolution (<1 μm). Therefore, coupling SILK to NanoSIMS will result in the direct measurement and imaging of individual plaques towards Alzheimer’s plaques at the nanometer level.
You are here:
home » Alzheimer’s plaques imaging