Summary: Proton therapy has emerged as one of the most advanced forms of radiotherapy, offering the ability to deliver high doses of radiation directly to tumours while sparing surrounding healthy tissues. Originating from a 1946 proposal by physicist Robert R. Wilson, the technique leverages the unique physical behaviour of protons — specifically the Bragg peak — to achieve unmatched targeting accuracy. This article traces the development of proton therapy from its early experimental use in research laboratories to its current role in treating a range of cancers, particularly those in sensitive anatomical regions and in paediatric patients. It examines the underlying physics, clinical applications, advantages over conventional X-ray radiotherapy, as well as the significant challenges of cost, accessibility, and health equity. Technological innovations, including adaptive therapy, artificial intelligence–enhanced planning, and compact delivery systems, are discussed alongside future research directions. While proton therapy remains a costly and limited resource, it represents a fusion of physics and medicine that could shape the future of cancer care if made widely accessible.
Keywords: Proton therapy, Bragg peak, radiotherapy, cancer treatment, precision medicine, adaptive therapy
Historical Context and Early Development
The origins of proton therapy date back to 1946, when Robert R. Wilson, an American physicist, proposed the use of accelerated protons for medical treatment. His insight was based on the Bragg peak phenomenon, which describes how protons deposit the majority of their energy at a precise depth in tissue before coming to rest. This offered an unprecedented opportunity for accurate tumour targeting without damaging healthy tissue located beyond the treatment site.
Early clinical work relied on adapting research particle accelerators rather than purpose-built facilities. In 1954, the Berkeley Radiation Laboratory in California treated the first patients using protons, and three years later, Uppsala University in Sweden followed suit. The collaboration between the Harvard Cyclotron Laboratory and Massachusetts General Hospital from 1961 onwards provided decades of data on the technique’s safety and effectiveness. In the Soviet Union, proton therapy began in Dubna in 1967 and at the ITEP facility in Moscow in 1969, which remains operational. Switzerland’s Paul Scherrer Institute pioneered proton therapy for eye tumours in 1984, setting new standards for precision.
The Physics of Proton Therapy
Proton therapy works by exploiting the Bragg peak, where the majority of the proton’s energy is released at a predetermined depth in tissue. This contrasts with conventional photon-based radiotherapy, where the radiation dose is delivered continuously along the beam’s entire path, including beyond the tumour. By calibrating the energy of the proton beam, oncologists can ensure the Bragg peak occurs within the tumour volume, delivering maximum damage to cancer cells while sparing surrounding healthy structures.
The earliest delivery method, passive scattering, used physical devices to broaden the beam for full tumour coverage. Modern systems now employ pencil beam scanning, guiding a narrow beam magnetically to “paint” the tumour with precision, and intensity-modulated proton therapy, which adjusts beam strength across different tumour regions for optimal dose distribution.
Clinical Applications
Proton therapy is particularly beneficial when tumours are located near critical organs or in paediatric patients, where reducing radiation to healthy tissue can prevent long-term side effects. In children, this approach can reduce the risks of growth abnormalities, hormonal disorders, and secondary cancers. Tumours at the skull base or along the spine can be treated without exceeding the tolerance of the brainstem or spinal cord. Eye tumours, such as uveal melanoma, are well-suited to proton therapy because of the precision needed to preserve vision in the unaffected eye.
In adult cancers, proton therapy is used for certain prostate cases, particularly where organ preservation is important, although evidence shows mixed advantages over advanced photon therapy in terms of long-term survival. Left-sided breast cancers may also be treated with protons to avoid heart damage. Increasingly, head and neck, brain, gastrointestinal, and liver cancers are being considered for proton therapy when anatomy or medical history makes dose sparing essential.
Comparing Proton and Photon Radiotherapy
Photon radiotherapy delivers a relatively high entrance dose, a therapeutic dose to the tumour, and an exit dose that continues into surrounding tissue. Proton beams, however, start with a lower entrance dose, deliver their peak within the tumour, and stop without an exit dose. This allows for equivalent tumour control in many cases, with reduced side effects in sensitive anatomical regions. However, in less critical sites, the difference in clinical outcomes may be minimal, and cost and accessibility become determining factors.
Side Effects and Quality of Life
Patients undergoing proton therapy often experience fewer acute effects such as fatigue, nausea, and skin irritation, as well as a reduced likelihood of long-term organ damage. Paediatric patients benefit most significantly from the reduced risk of developmental impairment and cognitive decline. Nevertheless, proton therapy is not entirely free from side effects, as the entry dose and scattered radiation can still affect surrounding tissues. Maintaining precision requires detailed imaging and planning, as even small anatomical changes during treatment can influence dose distribution.
Cost, Availability, and Infrastructure
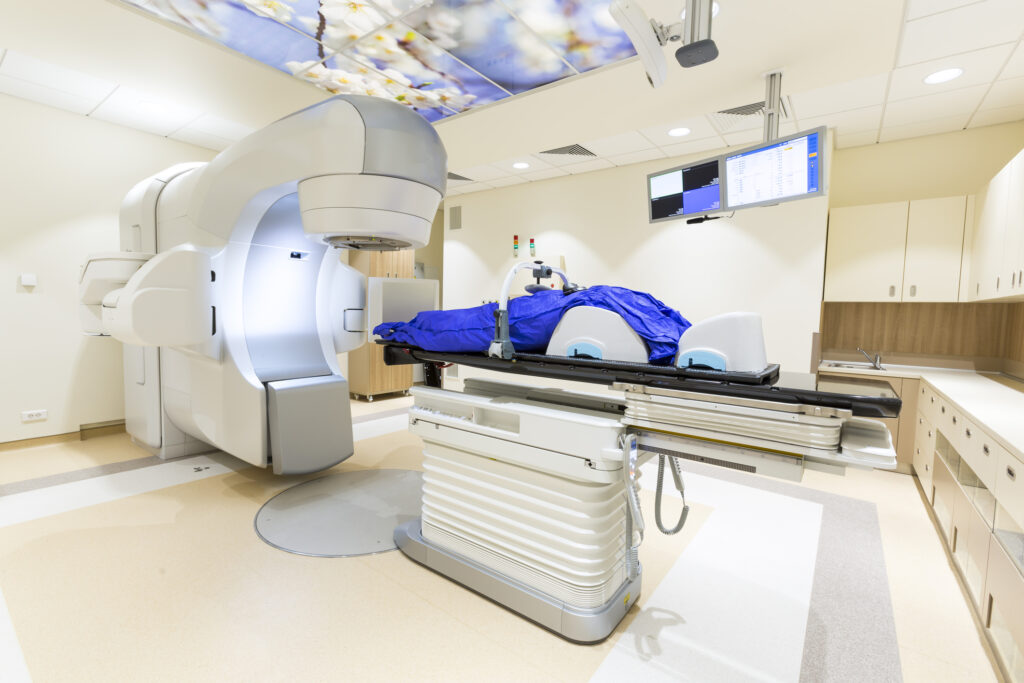
The complexity of proton therapy means it is one of the most expensive cancer treatments available. Building a full proton facility can cost in excess of £150 million and requires a large, highly trained team to operate. As of 2025, around 140 proton therapy centres exist worldwide, mainly in high-income countries. The NHS currently operates two centres in Manchester and London, but patients often have to travel considerable distances, and many countries have no access at all.
Health Equity and Access Challenges
Barriers to proton therapy are not only geographical but also financial. In countries without universal healthcare coverage, high treatment costs exclude many patients. Even where public funding exists, strict eligibility criteria can prevent access for those who might benefit. Studies have shown disparities in referral patterns, with socio-economic status, race, and age influencing who receives treatment. These inequities risk denying the therapy to patients most likely to benefit from it.
Technological Innovations
Recent research is addressing both the technical and economic limitations of proton therapy. Artificial intelligence has enabled rapid Monte Carlo dose calculations that once took hours, making adaptive planning more practical. Daily imaging combined with machine learning is paving the way for adaptive proton therapy, which adjusts treatment plans as tumours shrink or shift. Single-room, compact proton systems are being developed to reduce infrastructure costs, while FLASH proton therapy — delivering the full dose in a fraction of a second — is under investigation for its potential to further reduce harm to healthy tissue.
Future Directions
The future of proton therapy depends on technological refinement, cost reduction, and robust evidence from clinical trials. Ongoing research aims to determine which cancer types benefit most in terms of survival, toxicity reduction, and quality of life. Combining proton therapy with immunotherapy and molecularly targeted treatments could enhance its role in personalised oncology. As smaller, more affordable delivery systems become available, proton therapy could transition from being a rare, specialist service to a more widely accessible component of cancer care.
Conclusion
Proton therapy represents a powerful convergence of physics and medicine, capable of delivering cancer treatment with exceptional precision. For certain cancers, it offers clear advantages over conventional radiotherapy, particularly in protecting healthy tissue in sensitive regions and in treating children. However, its benefits remain unevenly distributed due to high costs and limited availability. The challenge for the coming years is to expand access without compromising the technological sophistication that makes the treatment effective.
From Robert Wilson’s theoretical proposal to the modern-day treatment of hundreds of thousands of patients, proton therapy has demonstrated both its potential and its challenges. If accessibility can be improved alongside continued innovation, it could become a cornerstone of cancer care in the decades ahead, delivering not only survival benefits but also a better quality of life for those facing a cancer diagnosis.
Disclaimer
The information provided in this article is for general educational and informational purposes only and is not intended as medical advice. Proton therapy and other cancer treatments should only be considered following consultation with a qualified healthcare professional who can assess individual circumstances. While every effort has been made to ensure the accuracy of the content at the time of publication, advances in research and clinical practice may lead to changes in understanding or recommendations. Open Medscience does not endorse any specific treatment, service, or healthcare provider and is not responsible for any decisions made based on the information contained herein.