Terbium radionuclides have moved from being a niche curiosity in radionuclide physics to a serious clinical proposition in nuclear medicine. Over the last two to three years, terbium-161 has reached formal phase I/II trials in prostate cancer, terbium-labelled somatostatin receptor ligands have entered the clinic, and the wider “terbium quartet” has been mapped out from production to preclinical applications.
This article walks through those developments, focusing on what they mean for theranostic practice.
The terbium quartet: one element, four theranostic tools
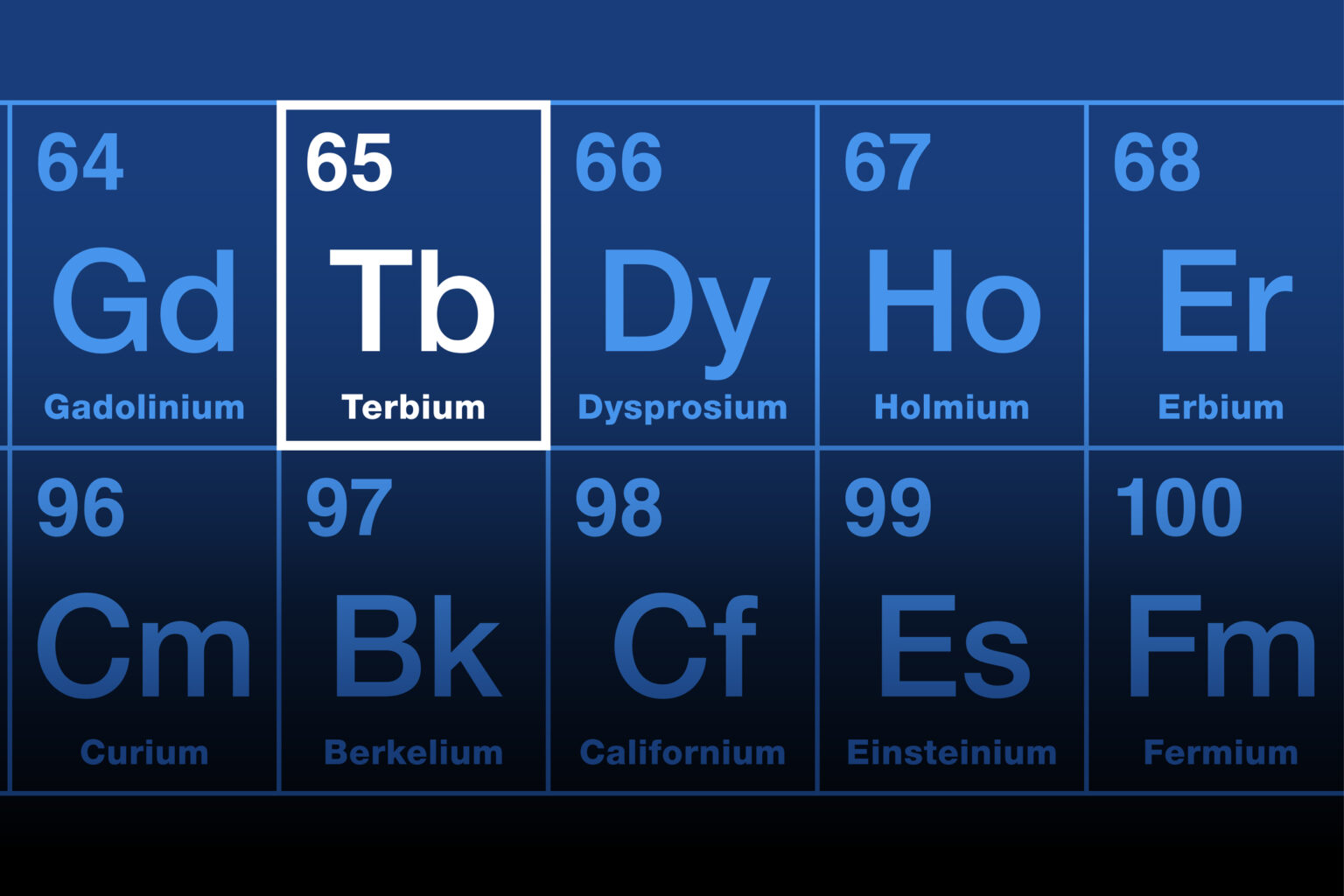
A key conceptual step was the systematic description of terbium’s four clinically interesting radionuclides in a 2024 Theranostics review: ¹⁴⁹Tb, ¹⁵²Tb, ¹⁵⁵Tb and ¹⁶¹Tb.
Each has distinct decay properties:
- ¹⁴⁹Tb is an α emitter with a small β⁺ component, making it a candidate for targeted alpha therapy with integrated PET.
- ¹⁵²Tb is a PET radionuclide (T½ ~ 17.5 h), suitable for whole-body imaging with the same chelators and vectors used for therapy.
- ¹⁵⁵Tb is a pure γ emitter (T½ ~ 5.3 d), ideal for SPECT imaging, dosimetry and extended biodistribution studies.
- ¹⁶¹Tb is a β⁻ emitter with a half-life of about 6.9 days, similar to lutetium-177, but with an additional cascade of low-energy conversion and Auger electrons that deposit dose over very short path lengths.
From a theranostic point of view, terbium offers a nearly “isotopic menu”: the same chemical scaffold and targeting vector can, in principle, be paired with an imaging radionuclide (¹⁵²Tb PET or ¹⁵⁵Tb SPECT) during work-up and dosimetry, and then switched to ¹⁶¹Tb or even ¹⁴⁹Tb for therapy. That conceptual flexibility is a big part of the enthusiasm around terbium, even though not all isotopes are yet clinically accessible.
¹⁶¹Tb: from phantom studies to the VIOLET trial
The most important step forward has been the clinical maturation of terbium-161. Preclinical work showed that ¹⁶¹Tb delivers more lethal hits to small clusters of tumour cells than ¹⁷⁷Lu, thanks to those extra low-energy electrons with path lengths in the micron range.
VIOLET: PSMA-targeted RLT in mCRPC
The VIOLET trial is the first prospective phase I/II study of a ¹⁶¹Tb-labelled PSMA ligand in men with metastatic castration-resistant prostate cancer (mCRPC). The protocol, published in 2024, outlined a single-centre, single-arm design using [¹⁶¹Tb]Tb-PSMA-I&T, with the primary focus on safety and secondary endpoints including PSA response, radiographic progression-free survival and quality of life.
In mid-2025, the first results were presented at ASCO and published in The Lancet Oncology. Thirty men with PSMA-positive mCRPC, who had already progressed on standard therapies, received up to four cycles of [¹⁶¹Tb]Tb-PSMA-I&T. Key outcomes included:
- A ≥50% decline in PSA in a large proportion of patients, with a meaningful fraction showing ≥90% PSA decline.
- Radiographic responses and progression-free survival figures that compare well with ¹⁷⁷Lu-PSMA data from earlier years, although cross-trial comparisons must be made cautiously.
- A safety profile broadly similar to ¹⁷⁷Lu-PSMA radioligand therapy (RLT), with haematological and salivary gland toxicities that appear manageable.
Dosimetry suggests that organ doses are similar to those for ¹⁷⁷Lu-PSMA, while modelling predicts higher cell-killing probability in micrometastases due to the ultra-short-range electrons. Clinically, this translates into hope that terbium-based PSMA RLT may better address early metastatic and minimal residual disease, where small clusters are the dominant problem.
The importance of VIOLET goes beyond its numerical outcomes. It demonstrates that GMP-grade ¹⁶¹Tb can be produced, that labelling to a common ligand such as PSMA-I&T is feasible, and that the regulatory pathway for terbium-labelled therapies can be navigated.
Beyond PSMA: terbium-161 in neuroendocrine tumours
Neuroendocrine tumours (NETs) were among the first indications where terbium-161 was explored, building on the success of ¹⁷⁷Lu-DOTATATE PRRT.
Back in 2021, a first-in-human feasibility study using ¹⁶¹Tb-DOTATOC in a patient with metastatic ileal neuroendocrine neoplasm demonstrated acceptable dosimetry and encouraging therapeutic effect. That experience laid the groundwork for further development.
A major step forward came in 2024 with the first-in-human administration of [¹⁶¹Tb]Tb-DOTA-LM3, a somatostatin receptor 2 antagonist, in a patient with hormone-active metastatic ileal NET. Antagonists like DOTA-LM3 occupy more receptor sites than agonists, and pairing this with ¹⁶¹Tb’s short-range electron emissions creates an attractive concept for maximising tumour dose.
In that case report, the authors showed:
- Strong tumour uptake on post-therapy imaging.
- Dosimetry compatible with safe administration to kidneys and bone marrow.
- Early clinical response with improvement in symptoms related to carcinoid syndrome.
These findings have fed into a formal clinical trial of ¹⁶¹Tb-DOTA-LM3 in NETs, currently underway in Basel in collaboration with the Paul Scherrer Institute (PSI), where the ¹⁶¹Tb is produced.
Parallel work continues with ¹⁶¹Tb-DOTATOC and ¹⁶¹Tb-DOTATATE. Preclinical studies suggest higher tumour dose delivery than ¹⁷⁷Lu analogues, with a particular advantage for small lesions and single cells. A recent industrial review from Isotopia Global summarised these data and argued for ¹⁶¹Tb-labelled somatostatin ligands as promising future standards for PRRT.
¹⁶¹Tb-PSMA beyond I&T: PSMA-617 and imaging
The PSMA story is not limited to PSMA-I&T. A pilot study of [¹⁶¹Tb]Tb-PSMA-617 in a patient with mCRPC who was previously resistant to ¹⁷⁷Lu-PSMA-617 demonstrated successful imaging and dosimetry, along with evidence of therapeutic effect. This provides proof that terbium labelling can be extended to the widely used PSMA-617 scaffold.
In addition, SPECT/CT imaging with ¹⁶¹Tb has been shown to be feasible, including whole-body scintigraphy and tomographic imaging for treatment monitoring and dosimetry. Standardisation of these techniques is still evolving, but from a practical point of view, centres familiar with ¹⁷⁷Lu SPECT will recognise much of the workflow.
Imaging partners: ¹⁵²Tb for PET and ¹⁵⁵Tb for SPECT
While ¹⁶¹Tb is leading on the therapeutic side, the diagnostic members of the terbium family are gradually entering use.
¹⁵²Tb has been produced at select centres and used in first-in-human PET studies, including ¹⁵²Tb-PSMA-617, which enabled high-quality imaging and biodistribution assessment with the same ligand used for therapy. Although ⁶⁸Ga and ⁶⁴Cu currently dominate PSMA PET, ¹⁵²Tb offers a matching half-life and chemical identity that can support more accurate dosimetry and kinetic modelling.
¹⁵⁵Tb is perhaps less well known, but it plays a key role as a pure γ-emitter for SPECT. Van Laere and colleagues highlighted ¹⁵⁵Tb as an attractive diagnostic partner for ¹⁶¹Tb, particularly for extended imaging protocols and personalised dosimetry without delivering a therapeutic β dose. For example, one could envisage an initial low-dose ¹⁵⁵Tb-labelled cycle for dosimetry followed by full-dose ¹⁶¹Tb therapy with the same ligand.
Both radionuclides remain limited by production capacity, but the concept of a fully isotopic terbium theranostic chain is now well defined.
¹⁴⁹Tb: α therapy on the horizon
The fourth member, ¹⁴⁹Tb, is attractive as an α emitter with a relatively simple decay scheme and no long-lived α-emitting daughters, which differentiates it from radionuclides such as actinium-225. Production, however, has been challenging.
Recent work at CERN-ISOLDE and other facilities has improved yields of ¹⁴⁹Tb through proton-induced spallation on tantalum and subsequent mass separation. These advances address one of the key bottlenecks: access to sufficient activities of ¹⁴⁹Tb with high radionuclidic purity for preclinical and, in time, clinical work.
Preclinical studies with ¹⁴⁹Tb-labelled tumour-targeting ligands are underway, but clinical translation is several steps behind ¹⁶¹Tb. The intriguing possibility is that a single terbium-labelled vector might eventually be available in both β⁻/Auger (¹⁶¹Tb) and α (¹⁴⁹Tb) formats, allowing escalation strategies similar to those explored with ¹⁷⁷Lu and ²²⁵Ac pairings today.
Production and supply: from boutique batches to trial-scale
For many years, terbium radionuclides were essentially research-only products, available intermittently from a small number of facilities. The current phase of development is characterised by attempts to scale up.
The Theranostics review and subsequent technical papers summarise the main routes: reactor-based production for ¹⁶¹Tb (typically via neutron irradiation of gadolinium precursors) and high-energy proton spallation combined with mass separation for ¹⁴⁹Tb, ¹⁵²Tb and ¹⁵⁵Tb.
Key facilities in this space include CERN-MEDICIS/ISOLDE, PSI, TRIUMF, and planned installations such as ISOL@MYRRHA, all working to improve yields, radiochemical processing, and logistics for the production of clinical-grade material.
On the industrial side, companies such as Isotopia Molecular Imaging are positioning themselves as suppliers of GMP-grade ¹⁶¹Tb for both NET and prostate cancer applications, which is essential if terbium RLT is to move beyond a small set of early-adopter centres.
Scaling is not just about more activity; it also demands robust quality control, long-distance transport solutions, and regulatory frameworks. Those are now starting to take shape, particularly around ¹⁶¹Tb, driven by the clinical trials mentioned above.
Practical implications for nuclear medicine
For clinicians and imaging scientists, terbium-161 is the isotope to watch in the short term.
In prostate cancer, ¹⁶¹Tb-PSMA-I&T has shown that it can deliver strong biochemical responses and promising progression-free survival in heavily pre-treated mCRPC patients, with a toxicity profile close to that of ¹⁷⁷Lu-PSMA therapies. If subsequent studies confirm improved control of micrometastatic disease, terbium-based PSMA RLT may move earlier in the treatment pathway.
In NETs, antagonists such as ¹⁶¹Tb-DOTA-LM3 combine high receptor occupancy with an emission profile well suited to killing single cells and small clusters. The early case reports and ongoing trial activity suggest that terbium PRRT could extend and refine the options opened up by ¹⁷⁷Lu-DOTATATE.
For imaging and dosimetry, ¹⁵²Tb and ¹⁵⁵Tb offer a future in which both diagnostic and therapeutic procedures share an identical chemical backbone. That may support more accurate kinetic modelling, more precise treatment planning and genuinely isotopic theranostic pairs.
From the laboratory perspective, the chemistry is straightforward for centres already comfortable with DOTA-based coordination and radiolabelling. The main learning curve lies in handling a new set of decay characteristics, optimising SPECT protocols for ¹⁶¹Tb and establishing dosimetry models that fully account for Auger and conversion electrons.
Looking ahead
Terbium radionuclides exemplify how nuclear medicine is shifting from “one isotope per indication” to a more flexible menu of decay schemes matched to tumour biology and disease stage. With ¹⁶¹Tb now supported by phase I/II data in mCRPC, first-in-human experience in NETs and a growing base of dosimetry and radiobiology, the element has clearly moved into the clinical era.
Over the next few years, the field will need:
- Head-to-head comparisons of ¹⁶¹Tb and ¹⁷⁷Lu regimens in both prostate cancer and NETs.
- Harmonised imaging and dosimetry protocols for ¹⁶¹Tb SPECT and, where feasible, ¹⁵²Tb/¹⁵⁵Tb imaging.
- Continued investment in isotope production and supply chains, particularly for ¹⁴⁹Tb and ¹⁵²Tb, to unlock the full theranostic potential of the terbium quartet.
For clinicians, physicists and radiochemists, terbium offers a scientifically elegant and increasingly practical toolset. For patients, especially those with small-volume or early metastatic disease, it may mark the start of a new generation of targeted radionuclide therapies designed not just to treat macroscopic lesions, but to seek out and destroy cancer at the microscopic level.
Disclaimer
This article is provided for informational and educational purposes only. It is not intended to offer medical advice, diagnose health conditions or recommend specific treatments. The scientific and clinical information presented reflects research and trial data available at the time of writing and may evolve as further evidence emerges. Readers should not rely solely on this content when making decisions about healthcare or clinical practice.
Any therapeutic approaches, radiopharmaceuticals or investigational agents discussed remain subject to regulatory review, local availability and professional oversight. Clinical use must always follow the guidance of qualified healthcare professionals, applicable laws and institutional policies.
Open MedScience does not endorse any specific product, service, radionuclide supplier or clinical protocol mentioned in this article. Neither the authors nor Open MedScience accept liability for actions taken based on the information provided. Always consult a suitably trained medical specialist before considering or altering any diagnostic or therapeutic intervention.
home » blog » radiotheranostics »