Summary: Magnetic Resonance Imaging (MRI) has become a cornerstone of modern diagnostic medicine, offering detailed images of the human body without the need for invasive procedures or ionising radiation. This article explores the fundamental scientific theory underpinning MRI, tracing its development from basic nuclear magnetic principles to its application in clinical settings. It discusses the physics of nuclear spin, resonance, magnetic field gradients, signal processing, and the role of contrast agents. The article also considers the importance of safety protocols and how future advancements may redefine the boundaries of medical imaging. Aimed at readers with a scientific interest or background, it demystifies the inner workings of one of medicine’s most powerful diagnostic tools.
Keywords: Magnetic Resonance Imaging, Nuclear Spin, Signal Processing, T1 and T2 Relaxation, Magnetic Fields, Diagnostic Imaging.
The Origins of MRI: From Physics to Medicine
Magnetic Resonance Imaging (MRI) has revolutionised the way we visualise the interior of the human body. Unlike X-rays or CT scans, MRI uses no ionising radiation. Instead, it relies on complex interactions between magnetic fields and atomic nuclei to produce high-resolution images. What might appear to be a relatively straightforward imaging modality is, in fact, underpinned by intricate physics, mathematics, and engineering.
The theory of MRI has its roots in the early 20th century with the discovery of nuclear magnetic resonance. In 1946, Felix Bloch and Edward Purcell independently observed that atomic nuclei in a magnetic field absorb and re-emit electromagnetic radiation at characteristic frequencies. This phenomenon—nuclear magnetic resonance—became the basis for spectroscopy in chemistry and later for medical imaging.
The jump from NMR spectroscopy to imaging occurred in the 1970s. Paul Lauterbur and Peter Mansfield introduced methods for spatial encoding of NMR signals, effectively enabling the construction of two- and three-dimensional images. For this work, they received the Nobel Prize in Physiology or Medicine in 2003. Their breakthroughs laid the groundwork for the MRI systems used in hospitals today.
The Science of Spin: What MRI Really Detects
MRI is fundamentally based on the magnetic properties of atomic nuclei, particularly the hydrogen nucleus, which is a single proton. Protons possess a property called spin, which gives rise to a small magnetic moment. In the absence of an external magnetic field, these magnetic moments are randomly oriented. However, when placed within a strong magnetic field, the protons tend to align either with or against the direction of the field.
The alignment process results in a net magnetisation vector along the direction of the magnetic field. This is the starting point for MRI signal generation. By applying a pulse of radiofrequency (RF) energy at the Larmor frequency—the specific resonance frequency of hydrogen nuclei within the field—the net magnetisation can be tipped away from its alignment. Once the RF pulse is turned off, the protons return to equilibrium, releasing energy in the process. This emitted energy is detected and forms the basis of the MRI signal.
Relaxation: T1 and T2 Mechanisms
The return of the net magnetisation vector to its equilibrium state is governed by two key processes: longitudinal relaxation (T1) and transverse relaxation (T2).
T1, or spin-lattice relaxation, refers to the time it takes for the magnetisation vector to realign with the external magnetic field. It involves energy exchange between the excited protons and their surroundings, or lattice.
T2, or spin-spin relaxation, refers to the loss of phase coherence among protons in the transverse plane. This occurs due to interactions between neighbouring magnetic fields generated by adjacent nuclei. T2 relaxation results in a decay of the MRI signal over time.
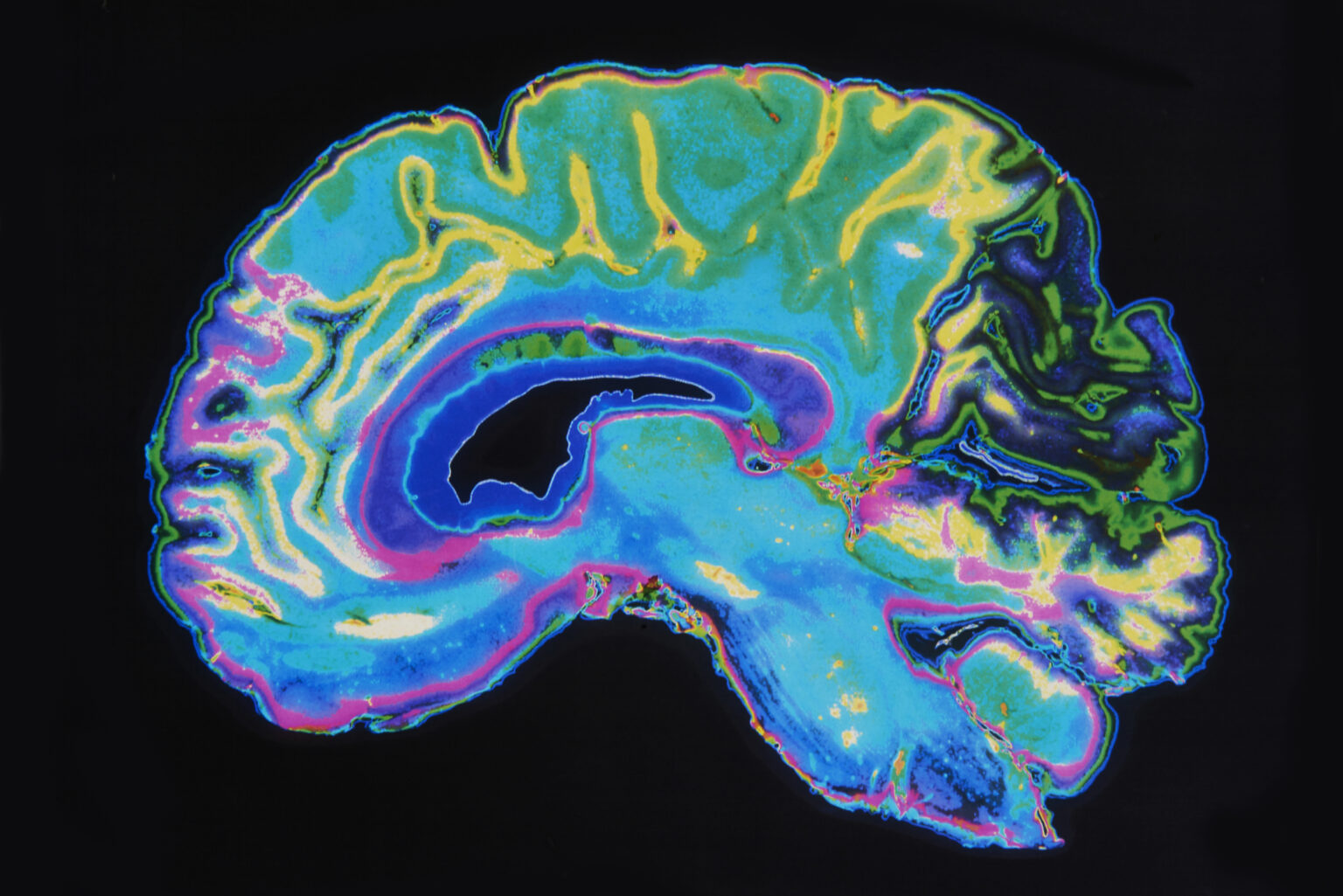
Different tissues have distinct T1 and T2 values. For instance, fat has a shorter T1 and T2 compared to cerebrospinal fluid. This difference allows MRI to produce contrast between various tissue types, making it particularly useful in neuroimaging and musculoskeletal diagnosis.
Magnetic Field Gradients and Spatial Encoding
One of the most ingenious aspects of MRI is the method by which spatial information is encoded into the NMR signal. This is achieved using magnetic field gradients—small variations in the magnetic field across different spatial directions (x, y, and z axes).
When a gradient is applied, the Larmor frequency of protons becomes dependent on their location within the magnetic field. By applying gradients in a controlled sequence, it becomes possible to determine where within the imaging volume the signal originates.
Three types of gradients are employed:
- Slice selection gradient: Determines the thickness and location of the imaging slice.
- Phase encoding gradient: Encodes spatial information in one in-plane direction by varying the phase of the signal.
- Frequency encoding gradient: Encodes spatial data in the orthogonal in-plane direction by varying the frequency.
Together, these gradients allow for the reconstruction of detailed two- and three-dimensional images using Fourier transform techniques.
Signal Detection and Image Reconstruction
The raw signals collected from the body are in the form of time-domain data, known as free induction decay (FID) signals. These signals are complex waveforms that represent the sum of electromagnetic waves emitted by precessing protons in various parts of the body.
The transformation of these signals into an image involves several mathematical processes. The most crucial among them is the Fourier transform, which converts the time-domain data into frequency-domain information. This frequency data can then be mapped back into spatial information using the known gradient encoding.
The final image is usually displayed as a matrix of pixels or voxels, where signal intensity corresponds to proton density and relaxation characteristics of the tissue. Advanced techniques, such as parallel imaging, compressed sensing, and machine learning algorithms, are being increasingly used to accelerate acquisition and enhance resolution.
Role of Contrast Agents
Although native tissue contrast in MRI is already high due to differences in T1 and T2 values, contrast agents are sometimes used to enhance image clarity and diagnostic accuracy. The most commonly used agents are gadolinium-based compounds. Gadolinium shortens the T1 relaxation time of nearby protons, making tissues that take up the agent appear brighter on T1-weighted images.
Contrast agents are particularly useful for identifying abnormalities such as tumours, inflammation, or blood-brain barrier breakdown. However, their use must be carefully considered due to potential side effects, especially in patients with impaired renal function.
Functional and Advanced MRI Techniques
Beyond structural imaging, MRI has evolved to include several advanced techniques that provide functional or physiological information. Examples include:
Functional MRI (fMRI): Measures changes in blood oxygenation to infer brain activity. It is widely used in neuroscience and pre-surgical planning.
Diffusion-Weighted Imaging (DWI): Detects the movement of water molecules in tissue. It is essential for the early detection of strokes.
Magnetic Resonance Spectroscopy (MRS): Provides metabolic information by measuring chemical concentrations in tissues.
Arterial Spin Labelling (ASL): Quantifies blood flow non-invasively without contrast agents.
These techniques extend MRI’s usefulness far beyond anatomical imaging, offering insights into cellular and physiological processes.
MRI Safety Considerations
While MRI is non-ionising and generally safe, it comes with specific risks. The powerful magnetic field can attract ferromagnetic objects with significant force, posing a danger to both patients and staff. Implants such as pacemakers, cochlear implants, and certain aneurysm clips may be contraindicated due to electromagnetic interference or the risk of heating.
Acoustic noise from gradient switching can exceed safe limits, necessitating the use of ear protection. Heating of tissue due to RF energy (quantified as Specific Absorption Rate or SAR) must also be carefully controlled, especially in long or high-field scans.
Safety protocols and proper screening are crucial to ensuring patient well-being during MRI examinations.
Future Directions in MRI Theory and Technology
MRI continues to evolve rapidly. High-field systems (7 Tesla and above) are being introduced into clinical use, offering unprecedented resolution but also requiring a refined understanding of magnetic field interactions.
Artificial intelligence and deep learning algorithms are being utilised to accelerate image acquisition, reduce noise, and facilitate automated diagnosis. Quantum computing, though still in its infancy, could eventually impact MRI signal processing and reconstruction.
On the theoretical side, developments in hyperpolarisation techniques, such as dynamic nuclear polarisation (DNP) and parahydrogen-induced polarisation (PHIP), promise to dramatically increase MRI sensitivity. These advances could open new avenues for molecular imaging and early disease detection.
Conclusion
Magnetic Resonance Imaging is a triumph of applied physics, blending complex theoretical principles with advanced engineering to produce images of remarkable clarity and detail. Its basis in nuclear magnetic resonance, coupled with innovations in gradient encoding, signal processing, and safety engineering, has made it an indispensable tool in modern medicine. As both technology and our understanding of human biology progress, MRI is likely to remain at the forefront of diagnostic imaging, continually shaped by the theory that gave it life.
By appreciating the scientific foundation of MRI, we not only gain a deeper respect for the technology itself but also a clearer understanding of its diagnostic capabilities, limitations, and future potential.
Disclaimer
The content provided in this article is intended for informational and educational purposes only. While every effort has been made to ensure scientific accuracy, Open Medscience does not offer medical advice, diagnosis, or treatment. The information should not be used as a substitute for consultation with qualified healthcare professionals or medical physicists. Readers are encouraged to verify any details that may affect clinical decision-making and to refer to professional guidelines or peer-reviewed sources where appropriate. Open Medscience assumes no responsibility for any consequences arising from the use or interpretation of the material presented.
[wpseo_breradcrumb]