Radiopharmaceutical diagnostics represent a pivotal intersection between nuclear medicine and diagnostic imaging, offering unparalleled insights into various diseases’ physiology and molecular biology. This discipline leverages radioactive substances, known as radiopharmaceuticals, to diagnose and sometimes treat diseases. Its applications span from cancer detection to cardiovascular, neurological, and other systemic conditions, providing critical information that influences patient management and therapeutic approaches. This overview explores the principles behind radiopharmaceutical diagnostics, its key applications, challenges, and the future direction of this evolving field.
Introduction
Radiopharmaceutical diagnostics stands at the forefront of medical imaging, harnessing the unique properties of radiopharmaceuticals to illuminate the human body’s inner workings in ways that traditional imaging techniques cannot. This innovative field combines the precision of molecular biology with the power of nuclear physics to offer a window into the physiological and pathological processes occurring within tissues and organs. By doing so, it transcends the limitations of conventional imaging modalities, which are often restricted to depicting the structural aspects of the body, offering a dynamic perspective that is as informative as it is transformative.
At the heart of radiopharmaceutical diagnostics are the radiopharmaceuticals themselves—compounds designed to achieve a delicate balance between specificity and safety. These substances comprise two fundamental components: a radioactive isotope, or radionuclide, which serves as the source of detectable radiation, and a pharmaceutical agent that guides the radionuclide to the intended biological target. The radiopharmaceuticals are introduced into the body, typically via injection, and then migrate to the areas of interest, where they emit radiation in the form of gamma rays or positrons. This emitted radiation is captured by sophisticated imaging devices, such as gamma cameras in single-photon emission computed tomography (SPECT) or positron emission tomography (PET) scanners, which convert these signals into detailed images of the body’s internal processes.
The utility of radiopharmaceutical diagnostics lies in its ability to provide vital functional insights into the body’s biochemistry. For instance, the glucose analogue Fluorodeoxyglucose (FDG) is used in PET imaging to identify areas of high glucose metabolism, a hallmark of many cancer cells. This allows for the early detection of malignancies, assessment of cancer spread, and evaluation of how well a treatment is working. Similarly, radiotracers like technetium-99m (Tc-99m) can be used to image blood flow to the heart muscle, enabling the diagnosis and management of coronary artery disease. The versatility of radiopharmaceuticals means that they can be tailored to explore a wide range of biological targets, from receptors and enzymes to proteins and cell markers, each offering a unique glimpse into various disease states and conditions.
The specificity with which radiopharmaceuticals can identify and bind to their targets is a testament to the remarkable advances in chemistry and molecular biology that underpin this field. This specificity ensures that the imaging is highly relevant to the pathological process being investigated and minimises the exposure of non-target tissues to radiation, thus adhering to the principle of radiation safety known as ALARA (As Low As Reasonably Achievable). Moreover, the quantitative nature of the images produced allows clinicians to detect disease presence and measure its extent and activity, providing a robust framework for making informed decisions about patient management.
Although, its profound capabilities, the development and application of radiopharmaceutical diagnostics are not without challenges. The production of radiopharmaceuticals requires sophisticated facilities capable of handling radioactive materials, and their use demands specialised knowledge and skills in nuclear medicine. Furthermore, due to their short half-lives, the perishable nature of many radiotracers necessitates seamless coordination between production, delivery, and administration to ensure optimal imaging results.
Today, radiopharmaceutical diagnostics represents a convergence of multiple scientific disciplines, each contributing to its growth and success. As it continues to evolve, it promises not only to enhance our understanding of disease mechanisms but also to revolutionise the diagnosis, staging, and monitoring of a multitude of conditions. By offering a detailed view of the body’s functional landscape, it empowers healthcare professionals to tailor treatments to patients’ individual needs, heralding a new era in personalised medicine.
Principles of Radiopharmaceutical Diagnostics
Radiopharmaceutical diagnostics intricately weaves the principles of radioactive decay with advanced imaging techniques to provide a profound insight into the body’s internal workings. This synergy between radiopharmaceuticals and imaging technology forms the cornerstone of nuclear medicine, enabling the visualisation of physiological processes that are otherwise invisible to conventional diagnostic methods. Understanding the fundamentals of radioactive decay and the mechanisms of imaging equipment is essential to appreciate the depth and breadth of radiopharmaceutical diagnostics.
Radioactive Decay in Diagnostics
At the core of radiopharmaceutical diagnostics lies the phenomenon of radioactive decay, a natural process by which an unstable atomic nucleus loses energy by emitting radiation. This decay process is integral to the function of radiopharmaceuticals, as the emitted radiation is the signal detected during imaging. The type of radiation emitted depends on the radionuclide used. For diagnostic purposes, gamma rays and positrons are of particular interest due to their ability to penetrate tissues and be externally detected.
Gamma rays are emitted during the decay of radionuclides, such as technetium-99m (Tc-99m), a workhorse of SPECT imaging. Conversely, positrons are emitted by radionuclides like fluorine-18, used in FDG, a common tracer in PET imaging. When a positron encounters an electron, they annihilate each other, producing a pair of gamma photons travelling in opposite directions. This event is crucial for PET imaging, as the simultaneous detection of these photons allows for precise localisation of the radiotracer within the body.
Imaging Techniques
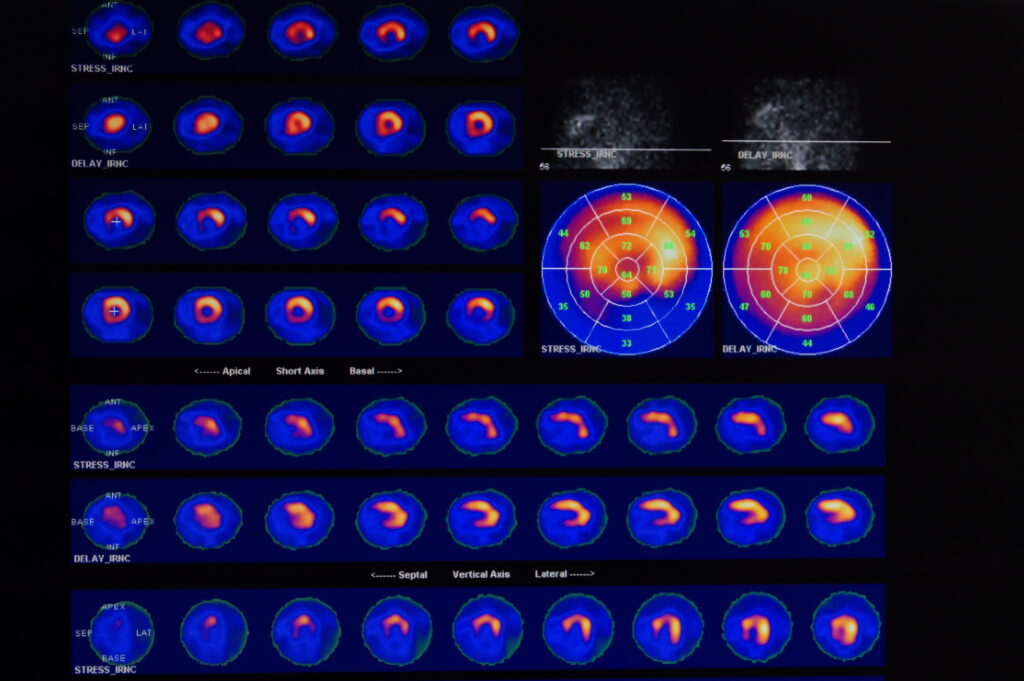
Gamma cameras are instrumental in detecting gamma radiation emitted by radiotracers during SPECT imaging. They consist of a detector head with a collimator. This device allows only gamma photons travelling in certain directions to reach the detector, thereby ensuring that the image represents the spatial distribution of the radiotracer. SPECT imaging provides three-dimensional images by rotating the gamma camera around the patient, offering detailed views of how radiotracers accumulate in organs and tissues.
PET scanners utilise the unique properties of positron-emitting radiotracers. Detecting coincident gamma photons resulting from positron-electron annihilation allows for creating images with high resolution and sensitivity. This technique is particularly valuable for detecting metabolic changes in tissues, such as the increased glucose metabolism in cancer cells using FDG-PET. PET imaging is used in oncology, cardiology, and neurology, providing critical insights into myocardial perfusion and brain metabolism.
Radiotracers in Diagnostics
The choice of radiotracer is pivotal, as it determines the specificity and utility of the diagnostic procedure. Radiotracers are designed to target specific physiological processes or molecular structures. FDG, for instance, is a glucose analogue taken up by glucose-using cells but trapped after phosphorylation, highlighting areas of high metabolic activity typical of cancerous tissues. Technetium-99m, due to its optimal radiation properties and half-life, is tagged to various compounds to image different organs and systems, from the skeletal system in bone scans to myocardial perfusion in heart disease.
The development and application of radiopharmaceuticals require a deep understanding of both the biological target and the principles of radioactive decay and imaging. This knowledge ensures that the radiotracers effectively reach and bind to their targets and emit detectable signals that can be accurately interpreted through SPECT or PET imaging. As a result, radiopharmaceutical diagnostics provides unparalleled insights into the body’s function and pathology, from the molecular level to whole-organ systems, guiding clinicians in diagnosis, treatment planning, and monitoring the efficacy of therapeutic interventions.
Key Applications of Radiopharmaceutical Diagnostics
Radiopharmaceutical diagnostics has emerged as a pivotal element in the multidisciplinary approach to medicine, offering unique insights into the diagnosis, staging, and treatment monitoring of diseases across oncology, cardiology, and neurology. By exploiting the properties of radiopharmaceuticals to target and visualise biological processes at the molecular level, this field has significantly enhanced our capability to detect and understand a wide array of conditions, leading to improved patient outcomes.
Oncology
In the area of oncology, the impact of radiopharmaceutical diagnostics is profound. Cancer, a complex and multifaceted disease, requires precise diagnostic tools for its detection, staging, and the assessment of treatment efficacy. PET imaging, particularly with fluorodeoxyglucose (FDG), plays a central role in this regard. FDG, a glucose analogue, is preferentially taken up by cancer cells due to their higher metabolic rate compared to normal cells. This differential uptake allows for the detailed visualisation of primary and metastatic tumours across a wide range of cancers, including lymphoma, melanoma, and various solid tumours.
The use of FDG-PET imaging has transformed the landscape of cancer care. It provides clinicians with detailed information about tumour metabolism, which is invaluable in distinguishing between benign and malignant lesions, thus aiding in the initial diagnosis. Moreover, FDG-PET is instrumental in cancer staging, offering a whole-body overview that can detect even small metastatic deposits often missed by conventional imaging techniques. This comprehensive detection capability is crucial for determining the appropriate treatment strategy, which may range from surgical intervention to systemic therapies.
Furthermore, FDG-PET imaging is indispensable in monitoring the response to treatment. Changes in the metabolic activity of a tumour, as evidenced by FDG uptake, can indicate the effectiveness of the treatment regimen, often before changes in tumour size become apparent. This early feedback allows for timely modifications to therapy, potentially improving patient outcomes. Additionally, FDG-PET can help identify recurrence, guide follow-up care, and provide surveillance strategies for cancer survivors.
Cardiology
In cardiology, radiopharmaceutical diagnostics, particularly myocardial perfusion imaging (MPI) with SPECT or PET, is a cornerstone in the evaluation of coronary artery disease (CAD), the leading cause of morbidity and mortality worldwide. MPI involves the use of radiotracers such as technetium-99m-labelled compounds in SPECT or rubidium-82 in PET to assess blood flow to the heart muscle. These tracers allow for the visualisation of areas with reduced perfusion, indicative of coronary artery blockages.
The ability to assess myocardial perfusion and function non-invasively provides invaluable information for diagnosing and managing CAD. MPI can help identify patients at high risk of cardiac events and guide treatment decisions ranging from pharmacological interventions to coronary revascularisation procedures. Additionally, cardiac radiopharmaceutical imaging can evaluate the viability of the heart muscle following a myocardial infarction, informing decisions about the potential benefits of revascularisation in patients with heart failure.
Neurology
The application of radiopharmaceutical diagnostics in neurology has opened new avenues for understanding and managing neurological disorders. PET imaging, using tracers designed to bind specific proteins or receptors, offers a window into the molecular pathology of conditions such as Alzheimer’s disease, Parkinson’s disease, and epilepsy.
In Alzheimer’s disease, PET tracers such as Pittsburgh compound B (PiB) target amyloid plaques, a hallmark of the disease, allowing for its early detection and aiding in the differentiation from other forms of dementia. Similarly, tracers that bind to tau protein aggregates provide insights into the progression of Alzheimer’s disease and other tauopathies. For Parkinson’s disease, radiotracers that visualise dopamine transporters can help in confirming the diagnosis and differentiating it from other movement disorders.
In epilepsy, PET imaging can localise seizure foci, especially in cases where surgical intervention is considered. Identifying the precise location of seizure origination can improve surgical outcomes, offering a chance for seizure freedom in patients with drug-resistant epilepsy.
The integration of radiopharmaceutical diagnostics across oncology, cardiology, and neurology exemplifies the transformative impact of this technology on modern medicine. By enabling the visualisation of diseases at the molecular and cellular levels, it not only aids in accurate diagnosis and staging but also provides a means to tailor and monitor treatment, embodying the principles of precision medicine. As research continues to advance, the development of new radiotracers and imaging techniques promises to expand the capabilities of radiopharmaceutical diagnostics further, offering hope for better outcomes across a broad spectrum of diseases.
Challenges and Limitations
Radiopharmaceutical diagnostics, while transformative, encounter several notable challenges and limitations that can impact its broader application and accessibility. The high cost associated with producing and procuring radiotracers, coupled with the sophisticated imaging equipment required for techniques such as PET and SPECT, poses significant financial burdens. These costs are often reflected in the healthcare expenses for patients and can limit the widespread adoption of these diagnostic methods, especially in resource-constrained settings.
Furthermore, the operation of radiopharmaceutical diagnostics demands specialised facilities equipped with the necessary safety measures to handle radioactive materials and highly trained personnel to conduct imaging procedures and interpret results. This requirement for specialised infrastructure and expertise can restrict the availability of these advanced diagnostic services to major medical centres, thereby limiting access for patients in remote or underserved regions.
Another critical concern is the radiation exposure associated with radiopharmaceuticals, which necessitates careful consideration and justification of their use, particularly in vulnerable populations such as children and pregnant women. Additionally, the short half-life of many radiotracers requires their production to be close to the point of use, often necessitating on-site or nearby cyclotron facilities. This logistical constraint can further limit the accessibility of radiopharmaceutical diagnostics, especially in areas lacking the necessary production capabilities.
Future Directions
The future of radiopharmaceutical diagnostics holds immense promise, driven by a convergence of advances in tracer development, imaging technology, and the burgeoning field of personalised medicine. Research efforts are intensely focused on the creation of new radiotracers that offer higher specificity to their biological targets, reduced toxicity to patients, and applicability across a wider range of diseases. Such innovations aim to refine the diagnostic accuracy, extend the utility of radiopharmaceutical diagnostics to earlier disease detection, and facilitate the monitoring of treatment responses with unprecedented precision.
Advances in imaging technology are poised to enhance radiopharmaceutical diagnostics capabilities significantly. Innovations in detector design, signal processing, and software algorithms are expected to improve the sensitivity and resolution of imaging systems like PET and SPECT scanners. These improvements will allow for the capture of finer details in the images produced, enabling clinicians to detect abnormalities at earlier stages and with greater accuracy. Moreover, advancements in quantitative imaging techniques are set to provide more precise measurements of tracer uptake and distribution, offering deeper insights into disease processes and treatment effects.
The integration of radiogenomics, which combines radiopharmaceutical diagnostics with genomic data, opens new avenues for personalised medicine. By correlating imaging findings with genetic information, clinicians can tailor diagnostic and therapeutic strategies to the unique molecular profile of an individual’s disease. This approach promises to enhance the effectiveness of treatments and minimise adverse effects by avoiding therapies unlikely to be beneficial for a specific patient.
Furthermore, the expansion of radiopharmaceutical diagnostics into personalised medicine will leverage computational methods and artificial intelligence to analyse complex datasets, integrating imaging, genetic, and clinical information to guide decision-making. This holistic approach is expected to revolutionise patient care, offering customised treatment plans that optimise outcomes while reducing unnecessary interventions and side effects.
The future of radiopharmaceutical diagnostics is bright, with ongoing research and technological advancements set to expand its role in early disease detection, accurate diagnosis, and the personalisation of healthcare, ultimately improving patient outcomes and quality of life.
Conclusion
Radiopharmaceutical diagnostics represents a frontier in medical imaging that merges the intricacies of chemistry, physics, and biological sciences with advanced imaging technology. This synergy has birthed a modality with the unparalleled capability to visualise and quantify biological processes within the living body, offering a window into the molecular underpinnings of disease. Its contributions to modern medicine are profound, facilitating early and accurate diagnoses, providing insights into disease progression, and informing tailored therapeutic strategies.
Despite facing challenges such as cost, accessibility, and the technical demands of managing radioactive materials, the field is rapidly advancing and innovating. Research and development efforts are continuously yielding new radiotracers with greater specificity and lower toxicity while advances in imaging technologies and computational analytics enhance the sensitivity, resolution, and quantitative analysis of diagnostic images.
The future of radiopharmaceutical diagnostics is bright, promising to extend its utility further into personalised medicine and beyond traditional boundaries. As it evolves, it holds the potential to revolutionise our understanding of complex diseases and to refine our approach to healthcare, ultimately leading to better patient outcomes and a deeper understanding of human biology. This dynamic field remains a cornerstone of precision medicine, embodying the promise of integrating scientific innovation with clinical care to improve lives.
Disclaimer
The content presented in this article is intended for informational and educational purposes only and does not constitute medical advice, diagnosis, or treatment. While Open Medscience has made every effort to ensure the accuracy and reliability of the information provided, it is not a substitute for professional medical guidance, and readers are encouraged to consult qualified healthcare professionals for any clinical concerns.
Radiopharmaceutical diagnostics and related imaging technologies discussed herein may involve the use of radioactive substances, specialised facilities, and regulated procedures. Their application should be carried out in compliance with applicable laws, regulations, and best practices by trained professionals. Open Medscience does not assume responsibility for any consequences arising from the use or interpretation of the information provided.
Views and opinions expressed are those of the authors and do not necessarily reflect the official policy or position of Open Medscience. Mention of specific technologies, radiotracers, or commercial entities does not imply endorsement or affiliation.
You are here: home » diagnostic medical imaging blog »