Radiotheranostics, an innovative and rapidly evolving field, integrates diagnostic imaging and targeted radiotherapy to enhance the precision and efficacy of cancer treatment. This article outlines the fundamentals of radiotheranostics, exploring its historical development, core components, and clinical applications. We examine the various current radiotheranostic pairs, discussing their mechanisms, benefits, and challenges. Furthermore, we consider this transformative medical discipline’s future prospects and potential advancements.
Introduction to Radiotheranostics
The convergence of diagnostic imaging and targeted therapy, known as radiotheranostics, represents a significant leap forward in personalised medicine. By combining these two aspects, radiotheranostics enables the simultaneous diagnosis and treatment of diseases, particularly cancers, with high precision and efficacy. This integrated approach improves treatment outcomes and minimises adverse effects, thus enhancing the overall quality of patient care.
Historical Development of Radiotheranostics
Radiotheranostics has its roots in the early 20th century when the therapeutic potentials of radioactive isotopes were first explored. The initial applications focused primarily on treating thyroid disorders with radioactive iodine. Over the decades, advancements in nuclear medicine and molecular biology paved the way for more sophisticated radiotheranostic approaches. The advent of positron emission tomography (PET) and single-photon emission computed tomography (SPECT) in the latter half of the 20th century marked a pivotal point, allowing for more accurate imaging and targeted treatment options.
Core Components of Radiotheranostic Pairs
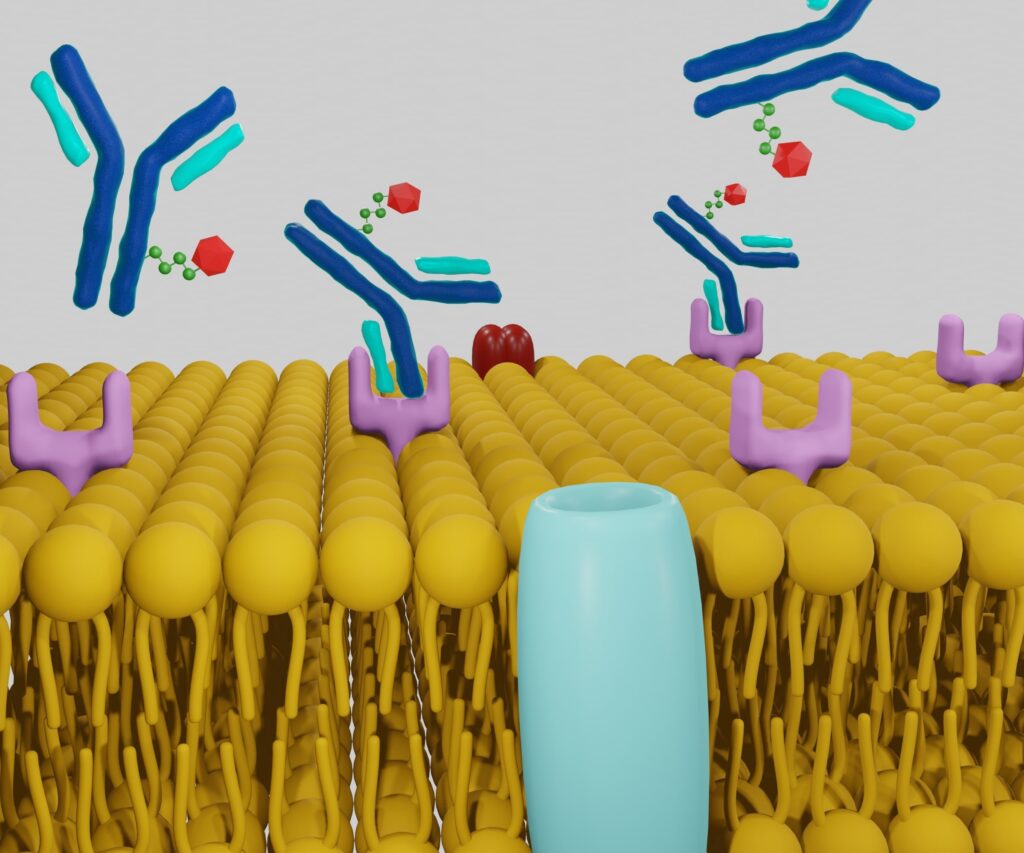
A radiotheranostic pair consists of two key components: diagnostic and therapeutic agents. These agents are typically linked to a targeting molecule, such as an antibody or a peptide, which binds specifically to cancer cells. The diagnostic agent, usually a radioactive isotope, enables precise imaging of the tumour, while the therapeutic agent delivers a cytotoxic dose of radiation to the cancer cells.
Mechanism of Action
The mechanism of radiotheranostic pairs involves the use of a targeting molecule that binds to specific biomarkers on the surface of cancer cells. Once bound, the diagnostic component emits signals that are captured by imaging technologies like PET or SPECT, providing detailed images of the tumour’s location and size. Following diagnosis, the therapeutic component, often a different isotope with cytotoxic properties, is administered. This isotope delivers targeted radiation to the cancer cells, destroying them while sparing surrounding healthy tissue.
Commonly Used Radiotheranostic Pairs
Several radiotheranostic pairs have been developed and are currently in clinical use. Some of the most notable pairs include:
Iodine-131 and Iodine-123
- Iodine-131: Used for both diagnosis and treatment of thyroid cancer and hyperthyroidism. It emits beta particles for therapeutic effects and gamma rays for imaging.
- Iodine-123: Primarily used for diagnostic imaging due to its favourable gamma emission properties and shorter half-life.
Gallium-68 and Lutetium-177
- Gallium-68: Used in PET imaging to detect neuroendocrine tumours. It provides high-resolution images that guide treatment decisions.
- Lutetium-177: Used therapeutically to treat neuroendocrine tumours, delivering targeted beta radiation to the tumour cells.
Technetium-99m and Rhenium-188
- Technetium-99m: Widely used in diagnostic imaging for various cancers, including breast and prostate cancer, due to its optimal imaging characteristics.
- Rhenium-188: Utilised for targeted radiotherapy, offering both beta and gamma emissions for therapeutic and imaging purposes.
Clinical Applications and Benefits
The integration of diagnostic and therapeutic modalities in radiotheranostics offers several advantages:
- Personalised Treatment: By tailoring the therapy based on precise imaging data, radiotheranostics enables personalised treatment plans that are more effective and less toxic than traditional methods.
- Early Detection and Monitoring: The high sensitivity of diagnostic agents allows for early detection of tumours and continuous monitoring of treatment response, facilitating timely adjustments to therapy.
- Minimised Side Effects: Targeted delivery of therapeutic agents reduces damage to healthy tissues, thereby minimising side effects and improving the patient’s quality of life.
- Improved Prognosis: Enhanced precision in diagnosis and treatment leads to better clinical outcomes and improved patient prognosis.
Challenges and Limitations
Despite the numerous benefits, radiotheranostics also faces several challenges:
- Complex Production and Handling: The production and handling of radiotheranostic agents require specialised facilities and expertise, limiting their availability to certain regions.
- Cost: The high cost of radiotheranostic procedures can be a barrier to widespread adoption, particularly in low-resource settings.
- Radiation Safety: Ensuring the safety of patients and healthcare providers from radiation exposure is crucial, necessitating stringent safety protocols and monitoring.
- Regulatory Hurdles: The regulatory approval process for new radiotheranostic agents can be lengthy and complex, potentially delaying their clinical application.
Future Prospects and Advancements
The future of radiotheranostics is promising, with ongoing research and technological advancements poised to address current challenges and expand its clinical applications. Some of the key areas of development include:
- Novel Targeting Molecules: Advances in molecular biology are leading to the discovery of new targeting molecules that can improve the specificity and efficacy of radiotheranostic agents.
- Improved Imaging Technologies: Innovations in imaging technologies, such as hybrid PET/MRI systems, are enhancing the resolution and accuracy of tumour imaging, enabling more precise treatment planning.
- Theranostic Nanoparticles: The development of theranostic nanoparticles that combine imaging and therapeutic functionalities in a single platform is an exciting area of research with the potential to revolutionise cancer treatment.
- Personalised Medicine: The integration of radiotheranostics with genomic and proteomic data is paving the way for more personalised and targeted therapies tailored to each patient’s tumour’s unique genetic profile.
- Artificial Intelligence (AI): The application of AI and machine learning in radiotheranostics is enhancing image analysis, treatment planning, and outcome prediction, thereby improving the overall efficiency and effectiveness of the treatment process.
Conclusion
Radiotheranostics represents a groundbreaking approach in the fight against cancer, offering a seamless integration of diagnosis and therapy that enhances the precision and efficacy of treatment. While challenges remain, ongoing research and technological advancements are continually improving the field, promising a future where personalised, targeted cancer treatments become the norm. As radiotheranostics continues to evolve, it holds the potential to significantly improve patient outcomes and transform the landscape of modern medicine.
Disclaimer
The information presented in this article is intended for educational and informational purposes only. Open Medscience does not provide medical advice, diagnosis, or treatment. The content herein is not a substitute for professional medical guidance, and readers are advised to consult qualified healthcare professionals regarding any medical concerns or treatment decisions.
While every effort has been made to ensure the accuracy and reliability of the information provided, Open Medscience makes no warranties or representations regarding the completeness, timeliness, or accuracy of the content. The article reflects the understanding and perspectives available as of the publication date and may not incorporate the most recent research or clinical guidelines.
Use of any information from this article is at the reader’s own risk. Open Medscience, its authors, and affiliated parties disclaim any liability for outcomes resulting from the application of the information contained in this publication.
home » blog » radiotheranostics »