Targeted Alpha Therapy uses radionuclides emitting alpha particles to precisely destroy cancer cells, minimising damage to surrounding healthy tissues.
Introduction to Targeted Alpha Radionuclide Therapy
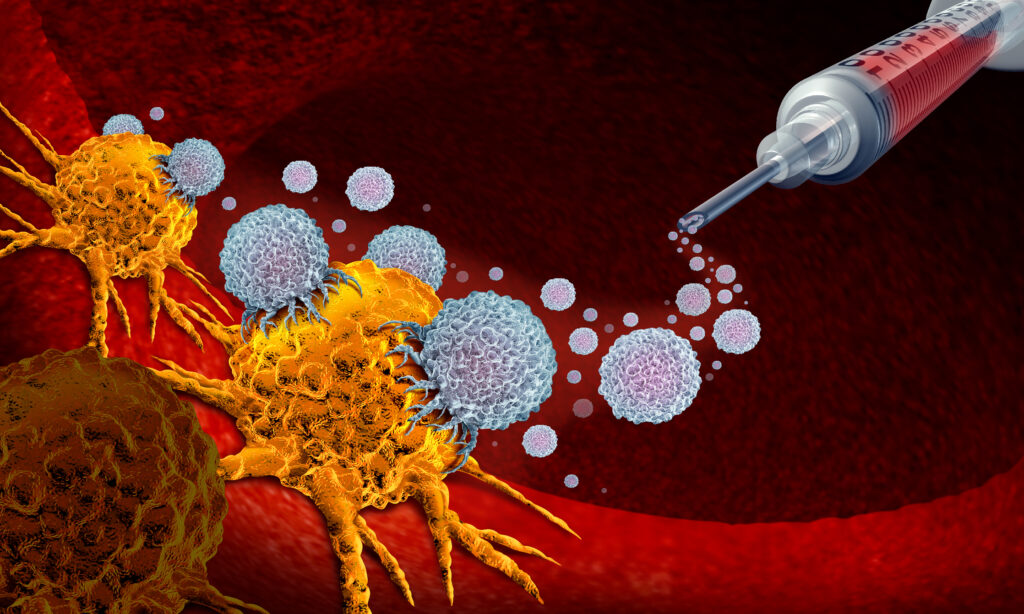
Targeted Alpha Radionuclide Therapy (TART) represents a cutting-edge approach in radiopharmaceutical therapy. It uses the potent cell-killing ability of alpha particles to treat microscopic tumours that are otherwise inaccessible through conventional methods. This innovative therapy is highly significant for its ability to target and destroy cancer cells while minimising damage to surrounding healthy tissue, using radionuclides chosen for their unique properties.
The core mechanism of Targeted Alpha Therapy involves alpha-emitting radionuclides, isotopes that release alpha particles during radioactive decay. These particles are highly energetic, heavy ions capable of causing substantial cellular damage. Due to their high linear energy transfer (LET) rate, alpha particles deliver a concentrated dose of radiation over a very short range, typically spanning only a few cell diameters. This allows for a highly localised therapeutic effect, reducing the potential radiation exposure to surrounding healthy tissues, which is particularly important in the treatment of cancers near sensitive structures.
The selection of radionuclides for Targeted Alpha Therapy is crucial and depends on their physical and chemical properties. Radionuclides such as actinium-225, bismuth-213, radium-223, and astatine-211 are commonly used due to their optimal decay characteristics and biological compatibility. For example, radium-223 mimics calcium to specifically target and bind to bone, making it ideal for treating bone metastases commonly seen in prostate cancer patients.
Delivering these radionuclides to tumour sites with precision involves complex biochemical engineering. The radionuclides are typically bound to molecules like antibodies or specific proteins with a high affinity for cancer cells. This ensures that the alpha-emitting substance is delivered directly to the tumour site, maximising the therapeutic impact while reducing systemic side effects.
Clinical applications of Targeted Alpha Radionuclide Therapy have shown promising results, particularly in the treatment of cancers that are difficult to target with beta radiation or surgical interventions. For instance, trials involving radium-223 have demonstrated significant improvements in survival rates for patients with metastatic prostate cancer. Moreover, ongoing research and trials aim to expand the use of TAT to other cancers, potentially improving patient survival and quality of life by reducing treatment-related complications.
As research into TAT continues to progress, there is a focus on enhancing the specificity of radionuclide delivery and expanding the list of targetable cancers. Advances in molecular biology and chemistry are likely to lead to the development of more effective targeting agents and radionuclides with improved decay profiles. The future of TAT lies in its potential to become a mainstream treatment modality, offering new hope for more effective management of resistant and metastatic cancers. This technology promises to redefine the boundaries of cancer therapy, emphasising precision and patient safety in its application.
Key Radionuclides in TAT
Among the many radionuclides available, only a select few are deemed suitable for therapeutic applications, especially in Targeted Alpha Therapy. These include actinium-225, astatine-211, bismuth-212, bismuth-213, lead-212, radium-223, terbium-149, and thorium-227. Each of these radionuclides possesses distinct physical characteristics that make them uniquely promising for use in medical treatments.
Actinium-225
Actinium-225 is an emerging radionuclide with significant potential in the field of Targeted Alpha Therapy (TAT). Its utility stems from its capability to emit multiple alpha particles during its decay process, a characteristic that makes it highly effective at killing cancer cells. This ability to deliver multiple destructive hits to targeted cells increases the probability of inducing irreparable damage, thereby enhancing the efficacy of the therapeutic intervention.
One of the most notable properties of Actinium-225 is its relatively long half-life of approximately 10 days. This duration is beneficial in clinical settings because it provides sufficient time for the radionuclide to be prepared, delivered, and absorbed by the target tissue before significant decay occurs. This window allows for the optimisation of logistics in treatment delivery, ensuring that the radionuclide retains its therapeutic potency by the time it reaches the cancerous site.
The actinium-225 decay process produces several daughter isotopes emitting additional alpha particles, contributing to the overall therapeutic effect. This cascade of emissions can lead to a greater localised dose within the tumour, maximising the damage to malignant cells while limiting exposure to surrounding healthy tissue.
The applications of Actinium-225 in medicine extend beyond its physical properties. It is commonly linked to various molecules such as antibodies, peptides, or small molecules, specifically targeting and binding to cancer cells. This targeted delivery system ensures that Actinium-225 is brought in close proximity to the cancer cells, allowing the emitted alpha particles to exert their maximum effect with high precision.
The ongoing research and development of Actinium-225-based treatments indicate promising advances in treating a range of difficult cancers, particularly those resistant to other forms of treatment. As more clinical trials are conducted, Actinium-225’s potential to improve treatment outcomes and survival rates for cancer patients continues to grow, underscoring its significance in the next generation of cancer therapeutics.
Astatine-211
Astatine-211, a member of the halogen group, holds a unique position in the periodic table as the rarest naturally occurring element. Its scarcity and potent radioactive properties make it an invaluable asset in the area of Targeted Alpha Radionuclide Therapy. Astatine-211 is particularly favoured in medical applications due to its optimal half-life of approximately 7.2 hours, which provides a suitable timeframe for therapeutic procedures without the long-term radioactivity risks associated with isotopes having extended half-lives.
Its emission of strong alpha particles enhances astatine-211 in TAT. These particles are highly effective in inducing double-strand breaks in DNA, destroying cancer cells. This capability makes Astatine-211 exceptionally proficient at targeting and eradicating smaller cancer clusters. The precise delivery of its alpha emissions maximises the therapeutic impact on malignant cells while minimising collateral damage to surrounding healthy tissues, a crucial consideration in cancer therapy.
The application of Astatine-211 is particularly beneficial in cases where cancerous cells are dispersed or in difficult-to-reach areas, scenarios often encountered with metastatic diseases. Its ability to be conjugated with molecules that specifically target cancer cells, such as antibodies or peptides, enhances its delivery to the exact location of the tumour cells. This targeted approach allows for the concentrated release of energy directly to the cancer cells, thereby increasing the effectiveness of the treatment and reducing the likelihood of side effects commonly associated with broader radiation therapies.
Moreover, the development and refinement of synthesis methods for Astatine-211 have been crucial in advancing its application. The synthesis involves irradiating bismuth with alpha particles in a cyclotron, a process that must be carefully timed and executed to optimise yield and purity. Ongoing research continues to explore new methodologies to improve the production and application of Astatine-211, promising further enhancements in its efficacy as a therapeutic agent in the fight against cancer.
Bismuth-212 and Bismuth-213
Bismuth-212 and Bismuth-213 are significant isotopes in Targeted Alpha Therapy due to their distinct decay properties and adaptability to various therapeutic scenarios. Each isotope brings specific advantages that make it suitable for different types of cancer treatments, highlighting the versatility of bismuth radionuclides in medical applications.
Bismuth-213 is particularly noted for its shorter half-life of about 46 minutes. This characteristic makes it ideal for rapid targeting and destruction of cancer cells. The brief half-life of Bismuth-213 allows for a quick decay that releases alpha particles powerful enough to cause lethal double-strand breaks in the DNA of cancer cells, thereby preventing their replication and leading to cell death. This rapid action is beneficial in acute therapeutic settings where a swift response to treatment is crucial, such as in circulating tumour cells or small, well-defined tumour masses that require immediate and localised intervention.
On the other hand, Bismuth-212 offers a longer half-life of about 60.55 hours, which makes it suitable for treatment scenarios that require a more prolonged duration to ensure the radionuclide reaches and affects the target tissue. The extended half-life provides a wider window for the radionuclide to travel through the body and accumulate in the targeted area, making it especially effective in treating larger or more diffuse tumorous areas. Bismuth-212 decays, emitting both beta and alpha radiation, giving it a dual therapeutic effect—initially, the beta particles help reduce the tumour size, and later, the alpha particles deliver a highly localised and potent dose to kill cancer cells.
Bismuth-212 and Bismuth-213 in Targeted Alpha Radionuclide Therapy are enhanced through their conjugation with antibodies or peptides that specifically target tumour cells. This targeting capability directs the isotopes precisely to the cancerous tissues, maximising therapeutic efficacy while minimising damage to healthy cells. The ongoing development in the chemical processing and conjugation techniques for these isotopes continues to improve their utility and effectiveness, making them critical tools in the expanding field of targeted cancer therapy.
Lead-212
Lead-212 is an intriguing radionuclide in the landscape of radiopharmaceutical therapy due to its unique decay characteristics. It emits both beta and alpha particles. This dual-emission property significantly enhances its therapeutic potential, enabling it to cater to a broader spectrum of cancer treatment scenarios and offering considerable flexibility in treatment planning.
The beta particles emitted by Lead-212 possess a moderate energy level, which allows them to travel a certain distance through tissues, making them effective for reducing the size of tumours by damaging cancer cells over a broader area. This initial reduction in tumour mass can be critical in managing larger tumours or those situated in less accessible regions of the body. Following the emission of beta particles, Lead-212 decays into Bismuth-212, which subsequently releases high-energy alpha particles. These alpha emissions are highly potent but have a much shorter range, delivering concentrated doses of radiation that can effectively kill cancer cells at a cellular level with minimal impact on adjacent healthy tissues.
This sequence of emissions — first, the broader-reaching beta particles followed by the localised, intense alpha radiation — allows for a staged approach to cancer treatment. Initially, the beta particles work to weaken and shrink the tumour, making the cancer cells more vulnerable to the subsequent targeted alpha attack. This one-two punch can be particularly effective in treating cancers that are resistant to conventional therapies, providing a robust method of attacking tumour cells.
The flexibility of Lead-212 in treatment planning lies in its capacity to be part of combination therapies and its suitability for varying sizes and types of tumours. Its use can be finely tuned according to the specific requirements of the patient’s condition, whether that involves more extensive initial tumour reduction or a more targeted approach. Moreover, the ability to control the dosing and timing of its decay products allows oncologists to optimise the therapeutic window, balancing efficacy with safety to minimise side effects.
Research and clinical trials continue to explore and expand the applications of Lead-212, with promising results that underscore its potential as a versatile and powerful tool in the fight against cancer.
Radium-223
Radium-223 is a standout radionuclide in Targeted Alpha Therapy, particularly renowned for its efficacy in treating bone metastases. As one of the most extensively researched radionuclides in TAT, its therapeutic value is predominantly attributed to its ability to mimic the behaviour of calcium, an essential element in bone physiology. This unique property allows Radium-223 to specifically target bone tissue, making it a potent tool against cancers that spread to the bones.
Bone metastases are a common and painful complication of various cancers, such as prostate and breast cancer, where the cancer cells spread from the primary tumour site to the bone. Conventional treatments like chemotherapy and external beam radiation can be less effective in these cases due to the complex nature of bone biology. However, Radium-223 directly addresses this challenge by homing in on bone tissues, integrating itself into areas of increased bone turnover typically seen in metastatic sites. Once incorporated into the bone matrix, Radium-223 begins its decay process, emitting powerful alpha particles.
The alpha emissions from Radium-223 have a very short range, typically less than 100 micrometres, which confines their cell-killing effects to a small radius. This highly localised action minimises damage to surrounding healthy tissue, focusing the therapeutic effects precisely where they are needed most. The high-energy alpha particles effectively induce double-strand breaks in the DNA of cancer cells, a form of damage that is particularly lethal and difficult for the cells to repair, leading to the targeted killing of bone metastases cells.
The clinical application of Radium-223 has shown significant benefits, including prolonged survival, improved quality of life, and better pain management for patients suffering from metastatic bone cancer. Its ability to provide targeted, effective treatment with minimal side effects has made Radium-223 a critical component of advanced cancer therapy protocols, particularly for patients with limited treatment options due to the location or extent of their disease. Continued research and clinical trials are further refining the use of Radium-223, promising ongoing improvements in outcomes for patients with bone metastases.
Terbium-149
Terbium-149 is a promising radionuclide in Targeted Alpha Therapy, though it is not as commonly utilised as some other isotopes, such as actinium-225 or radium-223. Its potential in clinical applications is attributed to its suitable half-life and decay properties, which are currently under rigorous investigation to determine their efficacy and safety in therapeutic settings.
Terbium-149 has a half-life of approximately 4.1 hours. This duration strikes a balance between being long enough to allow for the synthesis and administration of the radiopharmaceutical and short enough to limit the exposure of healthy tissues to radiation. This half-life enables precise control over therapeutic timing, allowing clinicians to schedule treatments that optimise the decay of Terbium-149 when it is most likely to be localised within the tumour.
The decay of Terbium-149 results in the emission of alpha particles, known for their high linear energy transfer (LET). High LET radiation is highly effective at damaging the DNA of cancer cells, leading to cell death. The energy released from the alpha particles emitted by Terbium-149 is substantial enough to destroy cancer cells. Still, it occurs over a short range, which helps to minimise the collateral damage to surrounding healthy tissues. This combination of powerful, localised radiation makes Terbium-149 an appealing candidate for TAT, especially for targeting small, localised tumours or those resistant to other forms of treatment.
The clinical research surrounding Terbium-149 focuses on maximising its therapeutic benefits while ensuring patient safety. Studies currently examine the best methods to chelate or bind Terbium-149 to molecules specifically targeting cancer cells, such as antibodies or peptides. These targeting molecules are designed to seek out and bind to antigens or receptors that are overexpressed on the surface of cancer cells, thereby delivering Terbium-149 directly to the tumour site.
One of the key challenges in working with Terbium-149 is the development of stable and efficient chelators that can hold onto the radionuclide until it reaches its target. The chelator must be strong enough to keep the Terbium-149 attached during circulation through the body but must also allow for its release when it has reached the cancer cells. Researchers are also studying the pharmacokinetics of Terbium-149 conjugates to understand how they distribute, metabolise, and are excreted from the body, which is crucial for minimising potential toxicity.
Safety studies are essential to the ongoing research, as with any radiopharmaceutical treatment. The radiological safety of Terbium-149, including its potential to cause harmful side effects, is being thoroughly evaluated in preclinical and early-phase clinical trials. These studies assess not only the direct effects of the radiation on tumour cells but also the potential for radiotoxicity in healthy tissues, particularly those near the treatment site.
Terbium-149 offers several advantages over other radionuclides used in TAT. Its alpha radiation is extremely effective at a cellular level, and its half-life allows for a more tailored therapeutic approach compared to longer-lived isotopes. These properties make Terbium-149 particularly suitable for outpatient therapy settings, where rapid decay and shorter treatment durations are beneficial both logistically and economically.
Furthermore, the ongoing development of new chelating agents and targeting molecules is expanding the potential applications of Terbium-149 in oncology. By engineering more specific and selective targeting mechanisms, researchers aim to increase the uptake of Terbium-149 by tumour cells, enhancing the therapy’s efficacy while reducing side effects.
The future of Terbium-149 in clinical oncology looks promising. As research progresses, new therapeutic protocols involving Terbium-149 are expected to be developed, potentially expanding its use beyond the current experimental settings into regular clinical practice. The ongoing improvement in chelating technologies and the better understanding of its pharmacodynamics are likely to pave the way for more effective and safer cancer treatments.
Terbium-149, though less commonly used, holds significant potential in the area of targeted cancer therapies due to its optimal decay properties and suitable half-life. Current studies are vital to establishing its role in TAT, with a strong focus on understanding its behaviour in the body, improving targeting efficiency, and ensuring patient safety. With continued research and development, Terbium-149 may soon become a key player in the fight against cancer, offering new hope to patients for whom conventional therapies have failed.
Targeted Alpha Radionuclide Therapy: Thorium-227
Thorium-227 is gaining prominence as a valuable radionuclide in the field of Targeted Alpha Therapy (TAT), recognised for its efficacy in treating large tumour masses. Its utility stems from a longer half-life and the emission of potent alpha particles, both of which are crucial for the effective management of more extensive and challenging cancers. The therapeutic impact of Thorium-227 is further enhanced by its decay products, which continue to contribute to the anti-tumour effects long after the initial administration.
Thorium-227 possesses a half-life of approximately 18.7 days, which provides a significant advantage when treating tumours that are large or have a complex vascular structure. This extended half-life allows for sustained radiation exposure at the tumour site, ensuring that the alpha particles have ample time to penetrate and destroy cancer cells throughout the tumour mass. The longevity of Thorium-227’s radioactivity means fewer treatment sessions are required compared to radionuclides with shorter half-lives, reducing the frequency of hospital visits and improving patient comfort and compliance.
The alpha particles emitted by Thorium-227 are highly effective in causing double-strand breaks in DNA, a type of damage that is particularly lethal to rapidly dividing cancer cells. This mode of action is beneficial for treating aggressive tumours that might not respond well to traditional chemotherapy or radiation therapy. The energy released by these particles is substantial, yet it is confined to a very short range, typically a few cell diameters. This localisation minimises collateral damage to surrounding healthy tissues, making Thorium-227 an ideal candidate for targeted therapeutic applications.
An additional advantage of using Thorium-227 in cancer therapy is the contribution of its decay products. As Thorium-227 decays, it produces a series of daughter isotopes, including radium-223, which itself is a proven therapeutic agent for bone metastases. Each of these isotopes also emits alpha particles, creating a cascade of therapeutic radiation that extends the efficacy of the initial treatment. This sequential decay ensures a prolonged release of alpha particles at the target site, enhancing the overall tumoricidal effect.
Thorium-227 is particularly valuable when the tumour mass is substantial, or the cancer has spread to multiple sites. For instance, its use in prostate cancer and breast cancer shows promising results, especially in cases where the disease has metastasised to bones. The ability of Thorium-227 to be conjugated to molecules that specifically target cancer cells, such as antibodies or peptides, allows for precise delivery of the radionuclide to the tumour, maximising therapeutic outcomes.
Current research focuses on expanding the use of Thorium-227 by developing new conjugates that can target a variety of cancer types. Ongoing clinical trials assess the safety and efficacy of these novel drug-radioisotope conjugates, with preliminary results showing favourable outcomes regarding tumour control and patient safety. The research also aims to understand the optimal dosing regimens and administration protocols to maximise the benefits of Thorium-227 while minimising potential side effects.
The promising results from ongoing studies and the unique properties of Thorium-227 suggest that it will play an increasingly significant role in the treatment of cancer. Its ability to effectively treat large tumour masses and provide prolonged therapeutic effects through its decay chain makes it a potent tool in the arsenal against cancer. As the medical community gains more experience and data from the use of Thorium-227, its application is expected to become more widespread, offering hope to patients with advanced and difficult-to-treat cancers.
Thorium-227 represents a pivotal development in the field of radiopharmaceuticals, offering enhanced capabilities for treating larger and more challenging tumour masses. Its longer half-life, potent alpha emission, and beneficial decay products collectively provide a robust platform for extending the reach and efficacy of Targeted Alpha Therapy.
Production and Chemical Behaviour
The production of radionuclides used in Targeted Alpha Therapy (TAT) is an intricate process that hinges on sophisticated technology and advanced infrastructure. The last twenty years have seen significant advancements in radionuclide production methods, largely propelled by the growing medical research and treatment demands. This period has witnessed improvements in the efficiency and safety of production techniques and a deeper understanding of the chemical behaviour of these radionuclides, which is crucial for their effective application in cancer therapy.
Advancements in Targeted Alpha Radionuclide Therapy Production
The production of radionuclides like actinium-225, thorium-227, and astatine-211 requires highly specialised facilities, such as nuclear reactors or particle accelerators, including cyclotrons. These facilities must be equipped with target stations and sophisticated control systems to manage the irradiation processes that produce radionuclides. For example, actinium-225 is generated through the irradiation of radium-226 or thorium-232 targets, which involves complex neutron capture reactions. Similarly, cyclotrons are used to produce astatine-211 by bombarding bismuth targets with alpha particles.
The complexity of these production methods is not just in the nuclear reactions involved but also in the subsequent chemical separation processes required to isolate and purify the radionuclides. These processes must be meticulously controlled to ensure the purity and quality of the radionuclides, as impurities can significantly affect both the safety and efficacy of the final therapeutic product. The development of automated and remotely operated chemical processing systems has greatly improved the safety and efficiency of these operations, minimising the exposure of technicians to radioactive materials.
Understanding the chemical behaviour of radionuclides is equally important for their application in TAT. This understanding helps in designing chelating agents and conjugate molecules that can securely bind the radionuclides and deliver them specifically to cancerous cells without affecting the surrounding healthy tissue. The chemical properties of each radionuclide determine how they can be attached to these carriers. For instance, the chemistry of radium-223, which mimics calcium, allows it to effectively treat bone metastases as it naturally homes to bone tissues.
Research into the coordination chemistry of radionuclides has led to the development of more stable and efficient chelators that can withstand the physiological conditions within the human body without releasing the radionuclide prematurely. This research is crucial because the radionuclide-chelator complex’s stability determines the therapy’s safety and effectiveness. Innovations in molecular targeting technologies have also allowed for the more precise delivery of radionuclides to tumour sites, enhancing therapeutic outcomes while reducing side effects.
Safely producing and handling radionuclides requires rigorous regulatory compliance and continuous monitoring to meet all safety standards. Facilities involved in producing and processing radionuclides must adhere to strict guidelines regarding radiation protection, waste management, and environmental safety. Additionally, the effectiveness of the radionuclides in clinical settings must be continuously assessed through clinical trials and research studies.
As the demand for radionuclides in medical applications continues to grow, further advancements in production technology and chemical research are expected. This could involve the development of new methods for producing radionuclides with improved yields and purities and the synthesis of novel chelating agents that can better target and treat a wider variety of cancers. The ongoing research and development in this field are driven by the ultimate goal of making TAT a more accessible and effective treatment option, thereby enhancing the quality of life and survival prospects for patients with cancer.
The production of radionuclides for TAT is a complex but critically important area of pharmaceutical sciences. It combines sophisticated nuclear and chemical engineering processes with advanced research in chemistry to deliver safe and effective cancer therapies. As technology and understanding of these processes continue to evolve, the role of radionuclides in medical treatments is set to become even more significant.
Biological Evaluation and Future Prospects
The biological evaluation of radionuclides in Targeted Alpha Therapy (TAT) is critical to developing effective cancer treatments. This process involves rigorous testing to assess the effectiveness and safety of radionuclides in clinical settings. Through these evaluations, researchers aim to understand how each radionuclide interacts with human biology, which is vital for optimising TAT treatments and enhancing patient outcomes.
The effectiveness of a radionuclide in TAT is primarily measured by its ability to selectively target and kill cancer cells without causing significant damage to surrounding healthy tissues. This selectivity is crucial for minimising side effects and improving the quality of life for patients undergoing treatment. Safety assessments focus on the potential toxic effects of radionuclides and their byproducts on human health, ensuring that the benefits of treatment outweigh the risks. These evaluations involve both in vitro studies, where the radionuclide’s effects on cancer cells are observed in a controlled environment, and in vivo studies, which test the radionuclide’s behaviour in the body, typically in animal models, before progressing to human trials.
Recent advancements in the biological evaluation of radionuclides have been promising, with an increasing number of clinical trials demonstrating improved patient outcomes. These trials are essential for testing the efficacy and safety of radionuclides under real-world conditions. They are often the final step before a treatment can be approved for general use. For instance, trials involving radium-223 have shown significant success in prolonging survival and reducing pain in patients with bone metastases from prostate cancer.
The future of TAT is looking particularly promising as ongoing research focuses on improving the specificity and efficiency of radionuclide delivery to tumour cells. Enhanced targeting is achieved through the development of novel molecular carriers that can more accurately guide radionuclides to their intended sites, thereby increasing the effectiveness of the treatment and reducing off-target effects. Additionally, advancements in imaging and diagnostic technologies allow for better tracking of radionuclide distribution and retention in the body, aiding in fine-tuning dosages and treatment schedules.
As our understanding of radionuclide materials and their interactions with human biology deepens, the potential for developing more effective and less harmful treatments for cancer increases. The integration of interdisciplinary research spanning chemistry, pharmacology, and oncology continues to push the boundaries of what is possible in TAT, heralding a new era of precision medicine in cancer therapy. With each breakthrough, TAT becomes more refined, potentially transforming the landscape of cancer treatment and offering new hope to patients facing this challenging disease.
Conclusion
The conclusion that the selective radionuclides used in Targeted Alpha Therapy (TAT) offer revolutionary prospects in cancer treatment is underscored by their unique blend of physical and chemical properties. These radionuclides are specifically engineered to hone in on and annihilate tumour cells, doing so with minimal impact on the surrounding healthy tissues—a cornerstone of what makes TAT so promising for oncology.
As these radionuclides are selectively targeted, they provide precision in previously unattainable treatment with older, more conventional therapies. This precision reduces the incidence and severity of side effects typically associated with cancer treatments, such as chemotherapy and traditional radiation therapy, which often affect healthy tissues as much as they affect tumour cells. Consequently, Targeted Alpha Therapy aims to be more effective and gentler on the patient, potentially transforming cancer treatment into a more manageable and less debilitating journey.
The ongoing research and development in the TAT field are set to significantly broaden its applications in oncology. Currently, TAT is used primarily for specific types of cancer, such as those that have proven resistant to other forms of treatment or have metastasised to locations like bones, where other treatments may be less effective. However, as research progresses, the potential to extend TAT to a wider array of cancer types looks increasingly feasible. This expansion of scope is particularly promising for patients suffering from cancers that are currently considered difficult or impossible to treat effectively.
Furthermore, the advancement of Targeted Alpha therapy represents a significant leap forward not just for therapeutic radiology but also for nuclear science as a whole. It exemplifies how nuclear science can be harnessed for profoundly beneficial applications in medicine, particularly in the innovation of treatments that can dramatically improve patient outcomes. The cross-disciplinary collaboration between nuclear physicists, chemists, oncologists, and pharmacologists highlights the importance of integrated scientific efforts in advancing medical science.
In conclusion, the continued evolution of TAT within the field of oncology not only offers hope to those affected by particularly challenging forms of cancer but also stands as a testament to the remarkable advances that can be achieved through the convergence of various scientific disciplines. With each development in the safety, efficacy, and specificity of radionuclide delivery, TAT moves closer to becoming a cornerstone of cancer treatment, potentially offering new life and new hope to patients around the globe. The trajectory of TAT is not just shaping the future of cancer therapy but also defining new frontiers in the application of nuclear science to medicine.
Disclaimer
The content presented in this article is intended for informational and educational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. The information provided reflects the current understanding and research in the field of Targeted Alpha Radionuclide Therapy (TAT) as of the publication date and may evolve with future scientific developments.
Open Medscience does not endorse any specific treatments, procedures, products, or opinions mentioned within the article. Readers are advised to consult with qualified healthcare professionals or medical practitioners before making decisions regarding their health or treatment options.
While efforts have been made to ensure the accuracy of the information, Open Medscience accepts no responsibility for any errors, omissions, or outcomes resulting from the use of this material. Any reliance placed on the content of this article is strictly at the reader’s own risk.
This article may reference ongoing research, clinical trials, or investigational uses of radiopharmaceuticals that may not yet be approved for widespread clinical use. Such information should not be interpreted as an endorsement or recommendation for use outside of approved regulatory pathways.
home » blog » radiotheranostics »