Summary: Targeted Radionuclide Therapy (TRT) is an innovative cancer treatment that uses radioactive isotopes to destroy cancer cells while selectively minimising damage to healthy tissues. TRT leverages the overexpression of specific cellular receptors on cancer cells, such as PSMA in prostate cancer and HER2 in breast cancer, to deliver radioactive payloads directly to tumours. The choice of radionuclides, such as beta emitters (e.g., Lutetium-177) and alpha emitters (e.g., Actinium-225), is crucial for effective therapy. Understanding radiation dosimetry and the tumour microenvironment further optimises the efficacy and safety of TRT. Ongoing advancements aim to enhance delivery, overcome resistance, and integrate TRT with other treatments, advancing personalised cancer care.
Keywords: Targeted radionuclide therapy; Cancer cells; Radioactive isotopes; Tumour microenvironment; Cellular receptors; Radiation dosimetry.
Introduction to Targeted Radionuclide Therapy
Cancer remains one of the foremost causes of mortality worldwide, necessitating the continual advancement of therapeutic strategies. Among these, Targeted Radionuclide Therapy (TRT) has emerged as a groundbreaking modality, offering the precision to eliminate cancer cells while sparing healthy tissues selectively. Unlike conventional treatments such as chemotherapy and external beam radiation therapy, TRT leverages biological interactions at the molecular and cellular levels. By exploiting the unique properties of radioactive isotopes, TRT delivers cytotoxic radiation directly to tumours, enhancing treatment efficacy and minimising adverse side effects. This article explores the biological principles that underpin TRT, encompassing cellular receptor targeting, radionuclide decay mechanisms, radiation dosimetry, and the dynamics of the tumour microenvironment. Understanding these principles is crucial for optimising the therapeutic potential of TNT and addressing the challenges associated with its application.
Role of Cellular Receptors
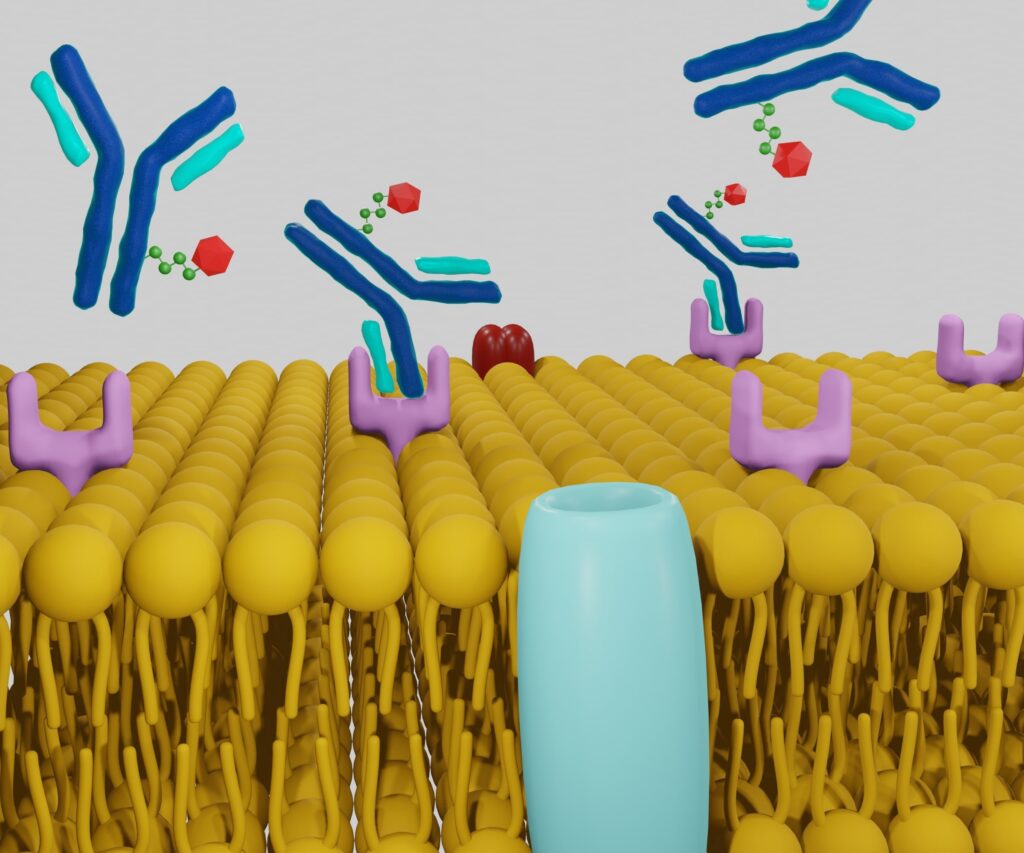
Central to the mechanism of TNT is the exploitation of cellular receptors that are uniquely or overexpressed on cancer cells. These receptors serve as anchoring points for therapeutic agents, ensuring the selective delivery of radioactive isotopes to malignant cells. For instance, prostate-specific membrane antigen (PSMA) is overexpressed in prostate cancer cells and serves as a prime target for TRT in prostate cancer treatment. Similarly, the human epidermal growth factor receptor 2 (HER2) is targeted in certain breast cancers.
The process begins with identifying ligands or antibodies that have a high affinity for these specific receptors. These ligands are conjugated with radioactive isotopes, forming a complex that binds selectively to cancer cells. The specificity of this binding is critical; it ensures that the radioactive payload is delivered predominantly to cancerous tissues, thereby minimising the exposure of healthy cells to radiation. This targeted approach not only enhances the efficacy of the therapy but also reduces the likelihood of systemic side effects commonly associated with non-targeted treatments.
Moreover, advancements in molecular biology have facilitated the engineering of highly specific antibodies and ligands with improved binding affinities and stability. This precision targeting is a cornerstone of TRT, enabling clinicians to customise treatments based on the receptor profiles of individual tumours, thereby advancing the field of personalised medicine.
Function of Radioactive Isotopes in Therapy
The efficacy of TRT hinges significantly on selecting appropriate radioactive isotopes, each characterised by distinct decay properties and emission types. Commonly used isotopes in TRT include Lutetium-177 (Lu-177) and Actinium-225 (Ac-225), which emit beta and alpha particles, respectively.
Beta emitters like Lu-177 release moderate energy over a relatively broad range (up to several millimetres in tissue), making them suitable for treating larger tumours. The beta particles induce ionising radiation that causes DNA damage in cancer cells, leading to cell death. Additionally, the longer path length of beta particles allows for a “crossfire” effect, where cells adjacent to the targeted cancer cells also receive radiation, enhancing the overall therapeutic impact.
In contrast, alpha emitters such as Ac-225 emit highly potent energy over a short range (a few cell diameters). This makes them particularly effective against micrometastases and isolated cancer cells, where precise, high-energy radiation can induce double-strand DNA breaks that are difficult for cells to repair. The limited range of alpha particles also ensures that surrounding healthy tissues are spared from significant radiation exposure, thereby reducing collateral damage.
The decay characteristics of these isotopes, including half-life and type of radiation emitted, are critical factors in determining their suitability for specific clinical scenarios. For instance, Lu-177 has a half-life of approximately 6.7 days, allowing sufficient time for the radiolabeled compound to accumulate in the tumour before significant decay occurs. In contrast, Ac-225 has a shorter half-life of about 10 days, necessitating careful timing in administration to maximise therapeutic efficacy.
Therefore, the choice of radionuclide is a strategic decision tailored to the tumour’s size, location, biology, and overall treatment plan. By matching isotopes with the specific needs of the cancer being treated, clinicians can optimise the balance between therapeutic benefits and potential risks.
Interactions with the Tumor Microenvironment
The tumour microenvironment (TME) is a complex and dynamic milieu surrounding cancer cells, consisting of stromal cells, immune cells, extracellular matrix components, and blood vessels. The TME plays a pivotal role in modulating the efficacy of TRT, influencing both the delivery of therapeutic agents and the sensitivity of cancer cells to radiation.
One of the critical factors within the TME is hypoxia, a condition characterised by low oxygen levels that is commonly found in solid tumours. Hypoxic conditions can reduce the efficacy of radiation therapy, as oxygen is a potent radiosensitiser that enhances the DNA-damaging effects of ionising radiation. Consequently, hypoxia can lead to radioresistance, necessitating strategies to overcome this challenge. Approaches such as combining TRT with hypoxia-activated prodrugs or using radionuclides that are less dependent on oxygen for their cytotoxic effects are being explored to mitigate the impact of hypoxia.
Another significant aspect of the TME is the extracellular matrix (ECM), which provides structural support to tumours. A dense ECM can impede the penetration and distribution of radiolabeled therapeutic agents, limiting their access to cancer cells. To address this, researchers are investigating methods to modify the ECM, such as using enzymes that degrade specific ECM components, thereby enhancing the delivery and distribution of TRT agents within the tumour.
Additionally, the TME includes various immune cells that can influence the response to TRT. The interplay between TRT and the immune system is an area of active research, with evidence suggesting that radiation can modulate immune responses, potentially enhancing the efficacy of immunotherapies. Understanding these interactions is crucial for developing combination therapies that harness the synergistic effects of TRT and immunomodulatory agents.
By comprehensively characterising and manipulating the TME, researchers and clinicians can significantly enhance the therapeutic outcomes of TRT, ensuring more effective and sustained cancer control.
Mechanisms of Cellular Damage
Once radiolabeled therapeutic agents are delivered to cancer cells via targeted receptors, the radioactive isotopes emit ionising radiation that induces cellular damage. The primary mechanisms through which this damage occurs involve the formation of DNA double-strand breaks (DSBs), which are particularly lethal to cells.
Alpha and beta particles, emitted by radionuclides used in TRT, interact with cellular components, leading to ionisation and excitation of molecules within the cell. This process generates free radicals, such as hydroxyl radicals, which can cause breaks in the DNA strands. DSBs are especially detrimental because they are challenging for cells to repair accurately, often leading to apoptosis (programmed cell death) or necrosis (uncontrolled cell death).
The efficacy of TRT is influenced by several factors related to cellular radiosensitivity. Cells in different phases of the cell cycle exhibit varying sensitivities to radiation; for example, cells in the G2/M phase are more susceptible to radiation-induced damage. Cancer cells’ intrinsic DNA repair capacity also plays a critical role in determining the response to TRT. Tumors with deficient DNA repair mechanisms are more likely to undergo cell death upon radiation exposure, making them ideal candidates for TRT.
Furthermore, the localisation of radioactive isotopes within the cell can affect the extent of damage. Radionuclides that localise near the nucleus or within the DNA can cause more effective DSBs than those that remain in the cytoplasm. Advances in molecular targeting strategies aim to direct radionuclides to specific intracellular compartments, thereby enhancing the cytotoxic effects on cancer cells.
Overall, the ability of TRT to induce irreparable DNA damage selectively in cancer cells while minimising harm to normal tissues underscores its potential as a highly effective cancer treatment modality.
Importance of Radiation Dosimetry
Radiation dosimetry—the quantitative measurement and calculation of radiation doses—is a fundamental aspect of TRT. Accurate dosimetry ensures that therapeutic radiation is delivered effectively to cancer cells while adhering to the safety thresholds of surrounding healthy tissues. This balance is critical for maximising treatment efficacy and minimising the risk of adverse effects.
Advanced imaging techniques, such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT), play a vital role in radiation dosimetry. These modalities enable real-time visualisation of the distribution of radiolabeled agents within the body, facilitating precise dose calculations. By integrating imaging data with mathematical models, clinicians can estimate the radiation dose delivered to both the tumour and normal organs, allowing for personalised treatment planning.
Personalised dosimetry approaches are increasingly crucial in TRT, accounting for patient-specific factors such as tumour size, location, metabolic activity, and individual pharmacokinetics. This tailored approach ensures that the administered dose is optimised for each patient, enhancing therapeutic outcomes and reducing the likelihood of toxicity.
Moreover, dosimetry is essential for monitoring treatment response and making necessary adjustments during therapy. By tracking the distribution and decay of radionuclides over time, clinicians can assess the effectiveness of TRT and modify dosing regimens accordingly. This dynamic approach to dosimetry contributes to the adaptability and precision of TRT, enabling ongoing optimisation based on real-time patient data.
In addition to clinical applications, dosimetry research continues to advance, with developments in computational modelling and machine learning algorithms promising even more accurate and efficient dose calculations. These innovations are poised to refine TRT further, enhancing its safety and effectiveness as a cancer treatment modality.
Challenges and Opportunities in TRT Development
Despite the significant advancements and promising outcomes associated with TRT, several challenges remain that necessitate ongoing research and innovation. Key challenges include ensuring the effective delivery of radioactive isotopes to tumour sites, overcoming resistance mechanisms, and minimising off-target effects that can lead to toxicity in healthy tissues.
Effective Delivery: Achieving uniform and sufficient delivery of radionuclides to the entire tumour mass is crucial for therapeutic success. Tumor heterogeneity, variations in receptor expression, and physical barriers within the TME can impede the distribution of TRT agents. Strategies to enhance delivery include developing multi-ligand targeting systems. These nanoparticles can navigate the TME more effectively and use external stimuli (such as ultrasound or magnetic fields) to facilitate deeper penetration of therapeutic agents.
Resistance Mechanisms: Cancer cells can develop resistance to TRT through various mechanisms, including upregulation of DNA repair pathways, alterations in receptor expression, and changes in cellular metabolism. Combination therapies integrating TRT with other treatment modalities, such as chemotherapy, immunotherapy, or inhibitors of specific signalling pathways, are being explored to combat resistance. These combinations aim to simultaneously target multiple aspects of cancer biology, reducing the likelihood of resistance development.
Minimising Off-Target Effects: Despite the targeted nature of TRT, some degree of radiation exposure to healthy tissues is inevitable. Off-target effects can lead to side effects such as bone marrow suppression, renal toxicity, and secondary malignancies. Advances in molecular targeting, radiolabel stability, and shielding strategies are essential for reducing these risks. Additionally, developing radioprotective agents that selectively shield healthy tissues while allowing radiation to affect cancer cells is an area of active research.
Theranostics: A significant opportunity in TRT development is the integration of theranostics—a combined diagnostic and therapeutic approach. Theranostics involves using the same or similar molecules for both imaging and therapy, enabling personalised treatment plans based on precise diagnostic information. For example, a diagnostic isotope can be used to visualise and quantify receptor expression and tumour burden, informing the selection and dosing of the therapeutic isotope. This seamless transition from diagnosis to treatment enhances the precision and effectiveness of TRT.
Novel Radionuclides and Targeting Mechanisms: The discovery and application of novel radionuclides with favourable decay properties and reduced toxicity profiles represent a promising frontier in TRT. Additionally, innovative targeting mechanisms, such as bispecific antibodies that can engage two different targets simultaneously or engineered ligands with enhanced specificity and affinity, are expanding the scope and applicability of TRT across various cancer types.
Clinical Integration and Regulatory Challenges: Integrating TRT into standard clinical practice involves navigating regulatory approvals, establishing standardised protocols, and ensuring access to specialised facilities for radionuclide production and administration. Collaborative efforts between researchers, clinicians, and regulatory bodies are essential for overcoming these hurdles and facilitating the widespread adoption of TRT.
Clinical Applications and Future Directions
TRT has demonstrated considerable success in treating a range of cancers, including neuroendocrine tumours, prostate cancer, and certain haematological malignancies. For example, Lu-177, labelled with somatostatin analogues, has been effectively used to treat neuroendocrine tumours, significantly improving patient outcomes. Similarly, PSMA-targeted TRT with Lu-177-PSMA has shown promising results in metastatic castration-resistant prostate cancer, offering a new avenue for patients with limited treatment options.
The future of TRT lies in its integration with other therapeutic modalities to enhance efficacy and overcome resistance. Combining TRT with immunotherapy, for instance, holds the potential to harness the immune system’s ability to recognise and eliminate cancer cells, thereby achieving synergistic effects. Additionally, integrating TRT with traditional chemotherapy or novel targeted therapies can provide comprehensive treatment strategies that address multiple facets of tumour biology.
Emerging research into novel radionuclides, such as alpha emitters with superior tissue penetration and reduced toxicity profiles, continues to expand the potential applications of TRT. Advances in molecular engineering also pave the way for more sophisticated targeting mechanisms, enabling the treatment of a broader array of cancer types with greater precision.
Personalised medicine is another critical direction for TRT, with treatments tailored to individual patients based on their tumours’ genetic, molecular, and physiological characteristics. Advances in genomic profiling and biomarker discovery facilitate the identification of patients who are most likely to benefit from TRT, thereby optimising treatment outcomes and minimising unnecessary radiation exposure.
Conclusion
Targeted Radionuclide Therapy represents a paradigm shift in cancer treatment, rooted in a deep understanding of biological principles that enable precise and effective targeting of malignant cells. By leveraging the specificity of cellular receptors, the unique properties of radioactive isotopes, and the intricate dynamics of the tumour microenvironment, TRT offers a highly innovative approach to cancer therapy. Accurate radiation dosimetry and the ability to induce irreparable cellular damage further enhance its therapeutic potential while minimising harm to healthy tissues.
Despite the challenges inherent in its development and application, ongoing research and technological advancements continue to address these hurdles, paving the way for broader clinical adoption and improved patient outcomes. The integration of TRT with other treatment modalities, the exploration of novel radionuclides and targeting mechanisms, and the move towards personalised medicine all contribute to the evolving landscape of TRT.
As our understanding of cancer biology deepens and our technological capabilities advance, Targeted Radionuclide Therapy is poised to play an increasingly prominent role in the arsenal against cancer, offering hope and improved quality of life to patients.
Disclaimer
The information provided in this article, Biological Principles Underpinning Targeted Radionuclide Therapy for Cancer, is intended for general informational and educational purposes only. It is not intended to be used as a substitute for professional medical advice, diagnosis, or treatment. While every effort has been made to ensure the accuracy of the content at the time of publication, Open Medscience makes no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, or suitability of the information contained herein.
The discussion of targeted radionuclide therapy (TRT), including its mechanisms, clinical applications, and future directions, reflects current scientific understanding and ongoing research. Clinical outcomes may vary, and medical decisions should always be made in consultation with qualified healthcare professionals who can consider an individual’s specific circumstances.
Open Medscience does not endorse any specific treatment, pharmaceutical product, or medical intervention discussed in the article. Any mention of radionuclides, biological targets, or commercial compounds is for illustrative purposes only and does not constitute an endorsement.
By reading this article, you acknowledge and agree that Open Medscience shall not be held liable for any loss, damage, or injury arising from the use or misuse of the information provided.
For medical concerns, please consult a qualified medical professional.
home » blog » radiotheranostics »