Tracing the Atom’s Legacy: The Evolutionary Journey of Radionuclides in Science and Medicine.
History of Radionuclides in Science and Medicine
The history of radionuclides is associated with the discovery of radioactivity and the subsequent exploration of the atomic nucleus. It has been a journey of scientific inquiry that has profoundly impacted medicine, industry, and our understanding of the universe.
The story of radionuclides begins with Henri Becquerel’s discovery of natural radioactivity in 1896. While experimenting with phosphorescent materials, he found that uranium salts emitted rays that could expose photographic plates, even without exposure to light. This observation hinted at a new kind of energy.
Marie and Pierre Curie were intrigued by Becquerel’s discovery and began researching uranium. They discovered that the intensity of the radiation was proportional to the amount of uranium, leading to the hypothesis that it was an atomic property. This research led them to discover two new elements in 1898: polonium and radium, which were far more radioactive than uranium.
Ernest Rutherford, often considered the father of nuclear physics, made significant contributions to understanding the structure of the atom and the principles of radioactivity. His gold foil experiment in 1911 led to the planetary model of the atom, with a dense nucleus at the centre. He identified alpha and beta radiation as helium nuclei and electrons, respectively.
Pioneering the Age of Radionuclides: From the Joliot-Curies’ Breakthrough to Medical Applications
The first artificial radionuclide was produced by Frederic and Irene Joliot-Curie in 1934. They bombarded aluminium with alpha particles to create phosphorus-30, which was radioactive. This discovery of artificial radioactivity opened the door to producing radionuclides, which had not existed on Earth before.
The first element without any stable isotopes, technetium, was artificially produced by Carlo Perrier and Emilio Segrè in 1937. Its metastable nuclear isomer, technetium-99m, discovered later, became the most widely used radionuclide in diagnostic nuclear medicine due to its ideal physical properties for medical imaging.
After the Second World War, nuclear reactors, initially developed for producing plutonium for weapons, began to be used for peaceful purposes, including the production of radionuclides for medical, industrial, and research applications.
In the 1940s, iodine-131 was one of the first radionuclides used in medicine. Because the thyroid gland absorbs iodine, I-131 proved to be effective in diagnosing and treating thyroid diseases.
The use of cobalt-60 in cancer therapy began in the 1950s. It was a critical step in the development of radiotherapy, providing a powerful and precise treatment option for tumours.
From PET and SPECT Imaging to the Dawn of Theranostics
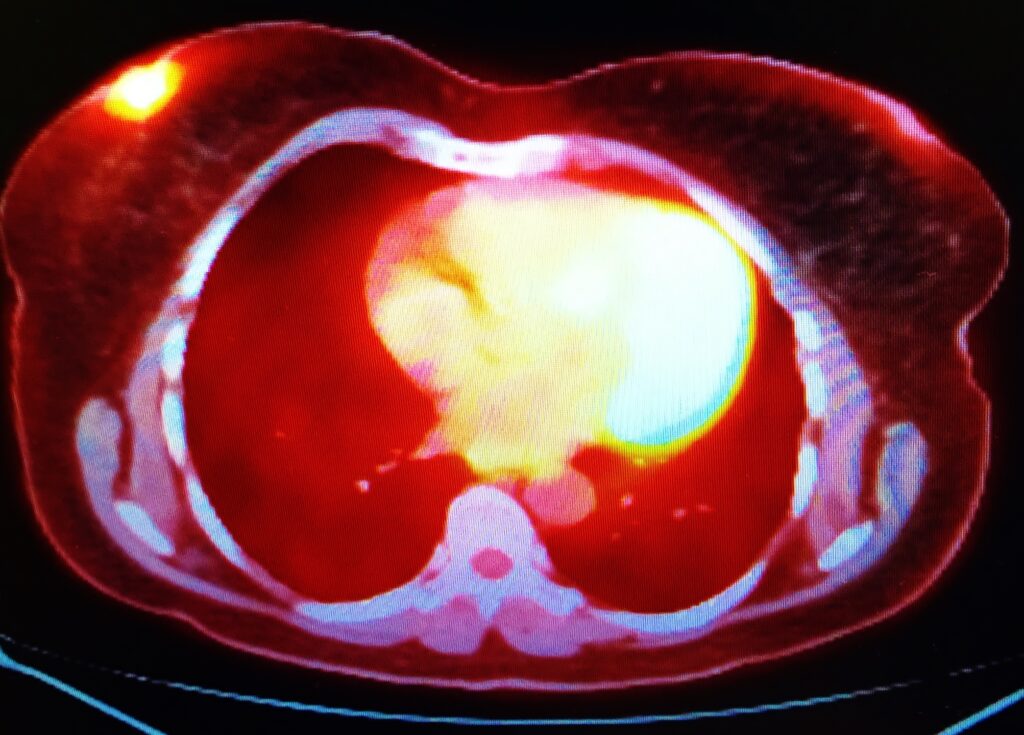
The advent of positron emission tomography (PET) and single-photon emission computed tomography (SPECT) in the 1960s and 1970s revolutionised medical imaging. Radionuclides such as fluorine-18 and technetium-99m enabled detailed, functional imaging of the human body, advancing diagnostics.
The chemistry of radionuclides advanced significantly in the late 20th century, with the development of new compounds that could target specific biological processes or structures in the body. This allowed for the creation of targeted radiopharmaceuticals for both imaging and therapy.
As the use of radionuclides grew, so did the understanding of the risks associated with radiation. Regulatory bodies were established to manage the safe use of radionuclides, and guidelines were developed for their handling, transportation, and disposal.
In the 21st century, the concept of theranostics emerged—using radionuclides for both therapy and diagnostics. This approach uses the same molecule labelled with different radionuclides to diagnose and treat diseases, particularly cancer.
The latest developments in radionuclide technology include new production techniques such as cyclotrons and linear accelerators, which allow for the creation of radionuclides with shorter half-lives and, therefore, less environmental impact. There is also an increasing focus on alpha-emitting radionuclides for targeted cancer therapy due to their high energy and short path length, which can destroy cancer cells more effectively and with less collateral damage.
In the second half of the 20th century, scientists began synthesising elements heavier than uranium (transuranium elements) in particle accelerators. These elements, including neptunium, plutonium, and others up to oganesson (element 118), are all radioactive, with many having no stable isotopes. These discoveries expanded the periodic table and deepened our understanding of nuclear reactions and stability.
Shaping the Future of Precision Medicine and Non-Invasive Diagnostics
Radionuclide imaging techniques have seen continuous improvement. Modern SPECT and PET scanners offer higher resolution, better sensitivity, and faster acquisition times. Hybrid imaging techniques, such as PET/CT and PET/MRI, have emerged, combining functional imaging with detailed anatomical images, significantly enhancing diagnostic accuracy.
The field of precision medicine has benefited from radionuclide research, allowing treatments to be tailored to the individual patient based on specific biological markers. Radionuclides are crucial in this personalised approach, particularly in identifying and targeting molecular pathways within tumours.
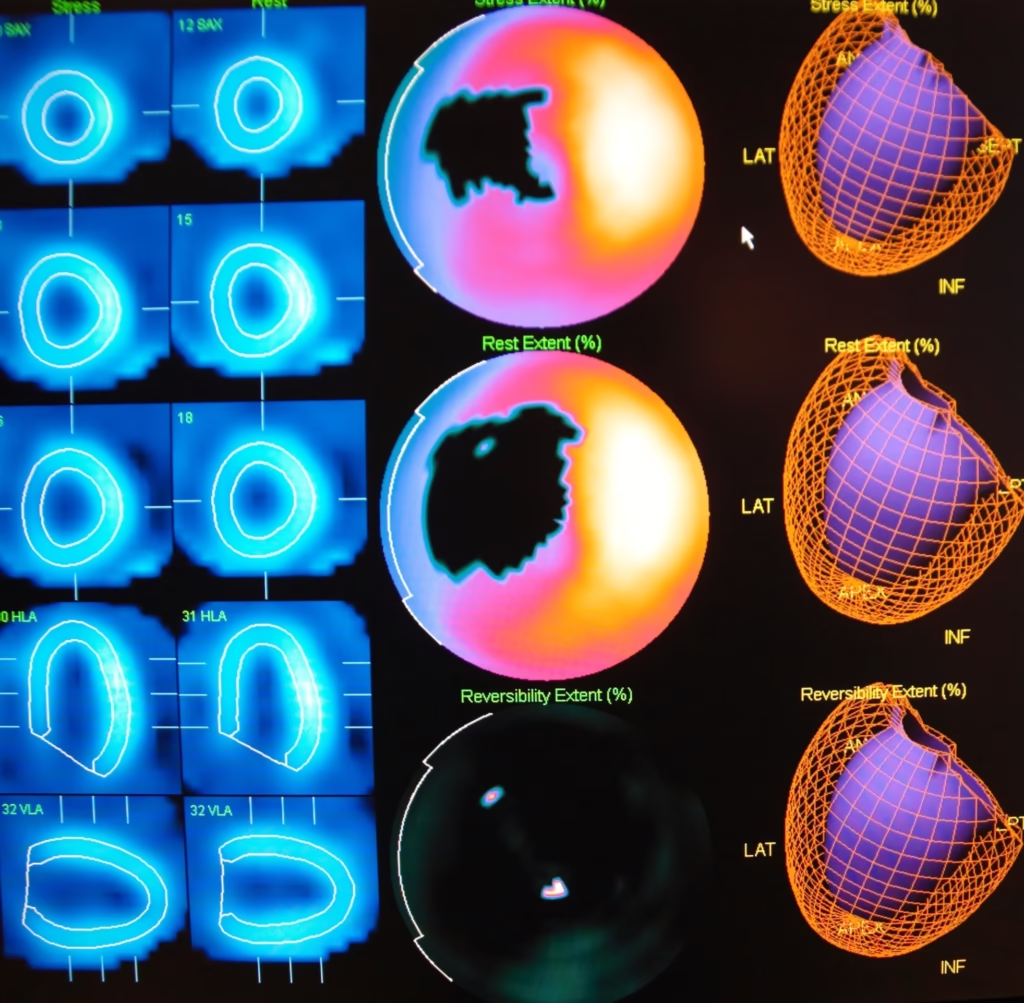
Radionuclides have facilitated the growth of non-invasive diagnostic procedures. For example, cardiac stress tests using radionuclides can assess heart function and blood flow without invasive catheterisation.
The International Atomic Energy Agency (IAEA) and various national agencies have developed frameworks for the safe use of radionuclides, ensuring that as new isotopes and applications are developed, they adhere to safety standards to protect both patients and healthcare workers.
The study of radionuclides has also enhanced the understanding of natural and anthropogenic radioactivity in the environment. This research has implications for environmental monitoring and has helped establish guidelines for acceptable levels of exposure from medical, industrial, and ecological sources.
Advanced Radiopharmaceuticals, Supply Challenges, and Therapeutic Innovations
The synthesis of radiopharmaceuticals has become more sophisticated, with chelating agents that can securely hold radionuclides, peptides that target specific receptors, and antibodies that seek out particular antigens on cancer cells.
Incidents like the Chernobyl disaster in 1986 and the Fukushima Daiichi nuclear disaster in 2011 have also influenced the study of radionuclides. These events have spurred developments in understanding radionuclide behaviour in the environment, their potential health impacts, and the need for emergency preparedness and response protocols.
As the 21st century progresses, one of the challenges in the field of radionuclides is ensuring a stable supply chain, especially for isotopes used in medicine. Ageing nuclear reactors and geopolitical issues can affect the availability of critical medical isotopes.
Efforts are underway to develop more sustainable and efficient methods of radionuclide production that do not rely on nuclear reactors. Innovations in accelerator technology and alternative production methods, such as neutron generators, are part of this trend.
Radionuclide therapy is expanding to include treatments for a broader range of conditions. Alpha-emitting radionuclides, with their highly localised radiation, are being investigated for their potential to treat micro-metastases without harming surrounding tissues.
Unlocking the Mysteries of the Atom: The Essentials of Radioactive Decay and Its Impact
Radioactive decay is a fundamental process by which an unstable atomic nucleus loses energy by emitting radiation. The term encompasses a variety of processes, including alpha decay, beta decay, gamma decay, and spontaneous fission. Understanding radioactive decay is crucial across many scientific domains, from astrophysics to nuclear medicine.
Alpha Decay occurs when an atomic nucleus ejects an alpha particle—consisting of two protons and two neutrons (essentially a helium-4 nucleus). This process decreases the mass of the original nucleus and changes it into a new element. Due to their relatively large size, alpha particles have a short range and are quickly absorbed by materials, including human skin, posing a hazard if radioactive materials are ingested or inhaled.
Beta Decay takes one of two forms: beta-minus decay, where a neutron is transformed into a proton with the emission of an electron and an antineutrino, or beta-plus decay (positron emission), where a proton becomes a neutron, releasing a positron and a neutrino. Beta particles can penetrate human skin but are stopped by protective clothing or a few millimetres of a substance like aluminium.
Gamma decay is a type of nuclear decay in which an excited nucleus releases energy in the form of gamma rays and high-energy photons. This decay occurs after other types of decay have occurred, and the nucleus is left in an excited state. Gamma rays are highly penetrating and can pose a serious health risk, requiring dense materials like lead for shielding.
Spontaneous fission is a form of radioactive decay in which a heavy nucleus splits into two lighter nuclei along with the emission of neutrons, gamma rays, and a large amount of energy. It is a rare event except in the heaviest elements.
Decay Chains occur when radioactive materials do not decay directly to a stable state but rather undergo a series of decays until they reach a stable isotope. These sequences of decays are known as decay chains or radioactive series.
However, the half-life of a radioactive isotope is the time required for half of the atoms in a sample to undergo decay. Half-lives can vary from fractions of a second to billions of years. The concept of half-life is key to the applications of radioactive isotopes, from dating archaeological finds (carbon-14 dating) to medical treatments (radiotherapy with isotopes like cobalt-60).
Radioactive Decay: A Keystone in Medical Innovation, Energy Production, and Scientific Discovery
- Medical: Radioactive decay is the basis of nuclear medicine, where radioactive isotopes are used for diagnosis and treatment. For example, iodine-131 is used for treating thyroid disorders, and technetium-99m is used for diagnostic imaging.
- Energy: The decay of uranium-235 and plutonium-239 is harnessed in nuclear reactors to produce energy.
- Scientific Research: Radioactive isotopes are used as tracers in biochemical research and environmental studies to track the movement of substances within a system.
- Rate of Decay: The decay rate of a radioactive substance is characterised by its decay constant, which is inversely proportional to the half-life. The decay rate is often expressed in terms of activity, which is the number of decays per unit time. The SI unit for measuring radioactive activity is the Becquerel (Bq), which is equivalent to one decay per second.
- Safety and Regulation: Due to the potential health risks associated with exposure to radiation, the handling and disposal of radioactive materials are strictly regulated. Safety measures include shielding, limiting exposure time, increasing distance from the source, and using personal protective equipment.
- Nuclear Stability and Decay Predictions: Nuclear stability is determined by the balance between the attractive nuclear force and the repulsive electromagnetic force within the nucleus. Nuclei that are too heavy or have an imbalance in the number of protons and neutrons are more likely to undergo radioactive decay. While the occurrence of decay for an individual atom is random, the decay process itself is subject to established statistical laws. Theoretical models can predict the likelihood of decay types and the stability of nuclei, which is vital in fields such as nuclear physics and engineering.
- Environmental Impact: Radioactive decay is a process that not only occurs in controlled settings but also happens naturally in the environment. Elements such as uranium and thorium decay slowly over geological timescales, contributing to the natural background radiation. Human activities, such as nuclear testing and reactor operation, have introduced additional radionuclides into the environment, which decay and impact ecosystems and human health.
- Cosmological Significance: Radioactive decay plays an essential role in astrophysics and cosmology. The heat produced by the decay of radioactive elements within Earth contributes to the geothermal gradient, which affects plate tectonics and volcanic activity. In space, the decay of isotopes within meteorites provides a valuable tool for dating the age of the solar system. The energy released by radioactive decay processes also influences the thermal evolution of planetary bodies.
- Isotopic Dating: One of the most significant applications of radioactive decay is in isotopic dating. By measuring the ratios of parent-to-daughter isotopes and knowing the half-life of the parent, scientists can date materials with a high degree of accuracy. Radiocarbon dating with carbon-14 is used for organic materials, while methods involving isotopes like potassium-40, uranium-238, and rubidium-87 are used for geological dating.
- Radiation Therapy: In cancer treatment, radioactive decay is exploited to damage the DNA of cancer cells more than that of normal cells, leading to the former’s death. Isotopes used in radiation therapy are chosen based on their type of decay and energy of emitted particles to maximise the therapeutic effect while minimising damage to healthy tissue.
- Nuclear Forensics: The study of radioactive decay also plays a pivotal role in nuclear forensics, which involves the analysis of nuclear materials to determine their origin and history. This is crucial in the context of nuclear security and nonproliferation.
Specific Activities of Radionuclides: Balancing Medical Efficacy and Safety in Diverse Applications
The specific activity of a radionuclide refers to the activity per quantity of atoms, typically measured in becquerels (Bq) per gram. It is a critical factor in the application of radionuclides, especially in medicine, industry, and research.
Radionuclides are atoms associated with an unstable nucleus that release energy in the form of radiation as they decay to become more stable. The rate at which they decay is defined by their half-life, which can range from fractions of a second to millions of years. The half-life influences the specific activity; radionuclides with shorter half-lives have higher specific activities since they decay more quickly.
The specific activity is significant in medical applications in diagnostic imaging and cancer treatment. For example, in Positron Emission Tomography (PET) imaging, radionuclides with high specific activity, such as Fluorine-18, are preferred. This is because a smaller amount of the radionuclide is required to achieve the necessary activity, reducing the mass of the compound that must be introduced into the body, thus minimising potential side effects.
Radionuclides with appropriate specific activity are used in therapeutic applications to target and destroy cancer cells. An example is Iodine-131, which is used in the treatment of thyroid cancer. The high specific activity allows for a concentrated dose to the cancerous tissue while limiting the exposure to surrounding healthy tissue.
The production of radionuclides with high specific activity often requires a cyclotron or a nuclear reactor. Cyclotrons are used to produce neutron-deficient radionuclides, which are commonly used in PET imaging. On the other hand, nuclear reactors are typically sources of neutron-rich radionuclides, which are useful in both imaging and therapy.
Radionuclides with high specific activities are used as molecular biology and biochemistry tracers in research. They allow for the tracking of molecules in the body or in biological systems, aiding in understanding metabolic pathways and molecular interactions.
Furthermore, in industry, specific activity can be a consideration in applications such as radiography, where it determines the intensity and penetration ability of the radiation, affecting the quality and speed of imaging.
Safety is paramount when handling radionuclides, especially those with high specific activities. Proper shielding, handling procedures, and disposal methods are crucial to protect individuals and the environment from the harmful effects of radiation.
As technology advances, the methods of producing radionuclides with specific activities that meet the demands of various applications continue to evolve. Research into new methods of production, such as accelerator mass spectrometry, holds the potential to create radionuclides with even higher specific activities.
Specific Activity of Radionuclides: Pivotal Role in Medical Diagnostics, Treatment, and Scientific Research
Medical isotopes are radioactive substances used to diagnose and treat various diseases, including cancer, heart disease, and neurological disorders. They play a crucial role in nuclear medicine, a field that combines chemistry, physics, biology, and medicine to develop diagnostic and therapeutic solutions.
Diagnostic Isotopes: In diagnostic imaging, medical isotopes are used in procedures such as PET and SPECT. These isotopes emit gamma rays or positrons upon decay, which are detected by special cameras to create detailed images of the body’s internal structures and functions.
| Radionuclide | Half-life | Type of Decay | Medical Use |
| Technetium-99m (Tc-99m) | 6 hours | Gamma | Diagnostic imaging |
| Iodine-131 (I-131) | 8 days | Beta and Gamma | Thyroid cancer treatment, diagnostic imaging |
| Fluorine-18 (F-18) | 110 minutes | Positron (Beta+) | PET imaging |
| Carbon-11 (C-11) | 20.4 minutes | Positron (Beta+) | PET imaging |
| Oxygen-15 (O-15) | 2 minutes | Positron (Beta+) | PET imaging, cardiac studies |
| Thallium-201 (Tl-201) | 3 days | Electron capture | Myocardial perfusion imaging |
| Lutetium-177 (Lu-177) | 6.65 days | Beta- | Cancer therapy |
| Radium-223 (Ra-223) | 11.4 days | Alpha | Treatment of bone metastases |
| Gallium-67 (Ga-67) | 3.26 days | Gamma | Tumor imaging, infection localization |
| Rubidium-82 (Rb-82) | 1.27 minutes | Positron (Beta+) | Myocardial perfusion imaging in PET |
| Yttrium-90 (Y-90) | 64 hours | Beta- | Radiation synovectomy, cancer therapy |
| Strontium-89 (Sr-89) | 50.5 days | Beta- | Treatment of bone pain from cancer metastases |
| Iodine-123 (I-123) | 13 hours | Gamma | Thyroid imaging |
| Copper-64 (Cu-64) | 12.7 hours | Beta+ and Beta- | Diagnostic imaging, therapy |
| Lead-212 (Pb-212) | 10.64 hours | Beta- and Alpha | Targeted alpha therapy |
| Astatine-211 (At-211) | 7.2 hours | Alpha | Targeted alpha therapy for cancer |
Advancements and Challenges in Medical Isotope Production: From Reactors to Regulatory Compliance
Production of Medical Isotopes: Medical isotopes are typically produced in nuclear reactors or cyclotrons. The most common production method involves irradiating a target material with neutrons in a reactor or with protons in a cyclotron, inducing a nuclear reaction that creates the desired isotope.
Supply and Demand: The supply of medical isotopes, especially Tc-99m, has been a concern due to the limited number of reactors capable of producing the parent isotope, molybdenum-99 (Mo-99). Efforts are ongoing to develop new production methods, including the use of non-highly enriched uranium (HEU) targets to address proliferation concerns.
Pharmacokinetics and Safety: When designing radiopharmaceuticals—compounds containing medical isotopes—scientists must consider pharmacokinetics to ensure that the isotope reaches the intended target in the body. The safety profile is also critical, as the isotope should decay within an appropriate timeframe to minimise radiation exposure to the patient.
Regulatory Oversight: Medical isotopes’ production, distribution, and use are highly regulated to ensure safety and efficacy. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), oversee the approval and monitoring of radiopharmaceuticals.
Research and Innovation: Researchers are continually seeking new isotopes with better properties, such as shorter half-lives and more suitable radiation types for imaging and therapy. Innovations in molecular biology and chemistry are expanding the applications of medical isotopes, allowing for more personalised and effective treatments.
Environmental Impact: The handling and disposal of medical isotopes are subject to stringent environmental regulations. Hospitals and medical facilities must have protocols in place to manage radioactive waste and ensure that it does not pose a risk to the environment or public health.
Conclusion
The specific activity of a radionuclide is a crucial property that determines its utility and application across various fields. It reflects the balance between the radioactive potency of the material and its practical quantity, impacting everything from patient safety in medical treatments to the precision of scientific research. As the need for radionuclides with specific activities grows, so does the importance of developing safe, efficient, and cost-effective methods for their production and utilisation.
The phenomenon of radioactive decay is a cornerstone of modern science and technology, with implications that stretch across numerous fields and applications. Its principles are fundamental to understanding processes ranging from the subatomic to the cosmological scale. The study and application of radioactive decay continue to be areas of dynamic research, with ongoing developments aimed at harnessing its power for beneficial uses while mitigating the risks associated with radiation exposure.
Medical isotopes are an indispensable tool in modern medicine, providing clinicians with powerful methods for diagnosis and treatment. As research advances, the field of nuclear medicine will continue to evolve, offering new isotopes and radiopharmaceuticals that enhance patient care and treatment outcomes. The future of medical isotopes lies in the development of safer, more effective, and more accessible isotopic applications, which will require continued innovation and collaboration across scientific disciplines.
The history of radionuclides is a testament to human curiosity and ingenuity. From the early days of Becquerel and the Curies to the modern era of nuclear medicine and theranostics, radionuclide research and application have evolved dramatically. This field has unlocked new frontiers in science and medicine and presented a paradigm shift in how we perceive and harness the atom’s power.
Disclaimer
The information contained in this article, “Radionuclides from Discovery to Modern Medical Imaging”, is intended for educational and informational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Readers should not rely solely on the content presented herein to make decisions about medical care or scientific practice.
Open MedScience has made every effort to ensure the accuracy and reliability of the information provided, drawing from reputable scientific and historical sources. However, the field of nuclear medicine and radiopharmaceutical science is continuously evolving, and some data may become outdated or superseded by new findings.
Any references to specific radionuclides, procedures, or therapeutic applications should not be interpreted as endorsements or recommendations for clinical use. Healthcare professionals and researchers are advised to consult relevant regulatory guidelines, peer-reviewed literature, and qualified specialists before applying any information from this article in clinical or research settings.
Open MedScience assumes no liability for any loss, injury, or damage incurred as a result of the use or misuse of the information provided in this article.
home » blog » nuclear medicine imaging »