Positron Emission Tomography (PET) has long been a cornerstone of molecular imaging, providing unique insights into physiology, biochemistry and disease at the molecular level. Recent advances in scanner design, radiotracer chemistry, artificial intelligence and clinical applications are rapidly reshaping the field. From total-body PET to novel tracers and AI-enhanced image reconstruction, these innovations are pushing molecular imaging into a new era of sensitivity, quantification and clinical relevance.
Expanding Horizons with Total-Body PET
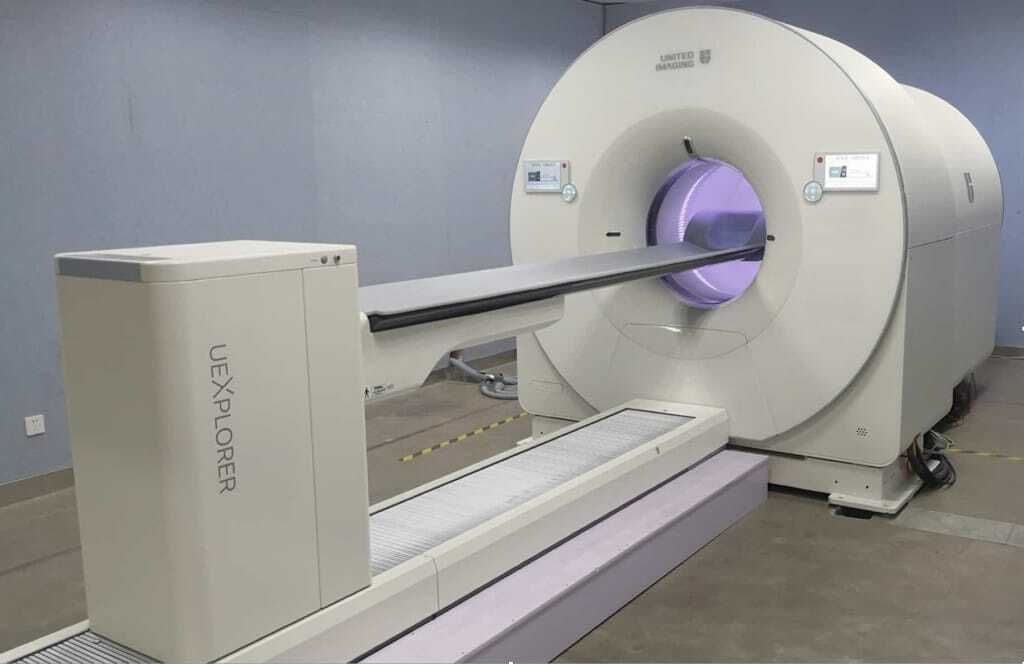
One of the most striking developments in PET technology is the emergence of total-body PET, also known as long-axial field-of-view (LAFOV) PET. Traditional PET scanners capture a section of the body at a time, requiring multiple bed positions to achieve full-body coverage. In contrast, total-body scanners—such as the EXPLORER and the United Imaging uEXPLORER—offer continuous coverage from head to toe, dramatically increasing sensitivity and data throughput.
This innovation enables simultaneous acquisition of signals from the entire body, providing a more complete view of tracer distribution and kinetics. The increase in sensitivity—up to forty times greater than standard PET/CT—enables shorter scan times, reduced radiation doses and the possibility of dual-tracer protocols. These scanners also enable new research applications, such as dynamic imaging of multi-organ interactions, whole-body pharmacokinetics, and the tracking of disease processes over time.
The UK’s National PET Imaging Platform has begun deploying total-body PET systems, with the University of Cambridge among the early adopters. These installations mark an important step towards establishing the country as a hub for advanced imaging research. For clinicians and radiochemists alike, this transition represents both an opportunity and a challenge. Data management, scanner calibration, and radiotracer validation for whole-body protocols all require new expertise and infrastructure.
In practice, total-body PET is expected to improve cancer staging, treatment monitoring and the study of systemic conditions such as inflammation and metabolic disorders. Its sensitivity also supports paediatric and longitudinal imaging, where lower radiation doses are particularly beneficial.
Hybrid PET Systems and Advanced Integration
Hybrid imaging has become increasingly important for both clinical and research applications. PET/CT remains the workhorse of oncology imaging, but PET/MRI systems are now making significant headway, especially in neurology and cardiology. The combination of PET’s molecular information with MRI’s soft-tissue contrast and functional imaging capabilities provides unmatched multiparametric data.
Recent developments include high-field (7 T) PET/MRI systems in preclinical research and ongoing refinements to time-of-flight technology and attenuation correction algorithms. Improved temporal and spatial resolution allows for better image fusion and more precise quantification. For radiopharmacy teams, hybrid systems introduce additional considerations: MRI attenuation correction, cross-modality calibration, and ensuring tracer stability and compatibility with MR environments.
The growing use of PET/MR also enhances translational research. Brain imaging, in particular, benefits from the ability to correlate molecular data with structural and functional MRI, enabling studies of connectivity, metabolism and receptor distribution in neurodegenerative and psychiatric disorders.
The Rapid Evolution of Radiotracers
While scanner technology attracts much of the attention, progress in radiotracer chemistry remains the lifeblood of PET imaging. The development of new tracers continues to expand PET’s reach beyond oncology into neurology, cardiology, infectious disease and inflammation.
Neurological Disorders
A major milestone has been the creation of tracers capable of imaging previously inaccessible molecular targets. A recent example is a tracer designed to detect nerve-fibre loss in multiple sclerosis lesions, providing a window into neurodegeneration that MRI alone cannot offer. Similarly, [18F]SynVesT-1 has demonstrated sensitivity to synaptic changes in spinal-cord injury and related brain regions, revealing neuroplasticity in response to trauma.
Another significant breakthrough involves tracers that bind to α-synuclein aggregates, a hallmark of Parkinson’s disease and related disorders. Until now, imaging this protein in vivo has been a major challenge. The ability to visualise α-synuclein could revolutionise early diagnosis and assessment of treatment efficacy in neurodegenerative disease.
Infectious and Inflammatory Diseases
PET imaging is also advancing in infectious disease research. A bacteria-specific tracer, [11C]PABA, was recently used to identify lung infection caused by Mycobacteroides abscessus, demonstrating the potential for pathogen-specific imaging. Beyond infection, new tracers targeting immune cell subsets, such as macrophages and lymphocytes, are being investigated for inflammatory and autoimmune conditions.
Oncology and Theranostics
Oncology remains the dominant application of PET, and the introduction of new prostate-specific membrane antigen (PSMA) tracers has markedly improved the detection of metastatic prostate cancer. The next generation of PSMA ligands aims to refine image contrast, reduce urinary background and extend utility to theranostic applications using matched therapeutic isotopes.
These advances in tracer design go hand-in-hand with evolving clinical guidelines. Updated “appropriate use criteria” for amyloid and tau PET now help clinicians determine when brain scans for dementia are warranted, balancing diagnostic benefit with cost and radiation exposure.
The expanding scope of radiotracers also has significant operational implications. Radiopharmacies must adapt synthesis routes, quality control protocols and regulatory submissions for each new compound. As the demand for multi-target and dual-tracer studies grows, coordination between chemistry, imaging, and clinical teams will become increasingly critical.
Artificial Intelligence and Data-Driven Imaging
AI is rapidly transforming image reconstruction, interpretation and workflow management in PET. Deep learning algorithms are being applied to reduce noise, improve spatial resolution and enable ultra-low-dose imaging. Recent research on generative super-resolution models using Fourier diffusion networks demonstrates the potential to recover high-quality images from limited data, reducing patient exposure and scan time.
Segmentation has also benefited from AI innovation. The “SegAnyPET” model, for instance, offers prompt-based universal segmentation across a wide range of PET datasets. Such tools streamline analysis, support multi-centre trials and facilitate the integration of PET data with other imaging modalities.
Beyond image reconstruction, AI is playing an increasing role in quantification and personalised dosimetry for theranostic applications. By integrating PET data with clinical and molecular information, AI models can predict tracer kinetics, optimise therapy dosing and monitor treatment response in real time. This shift towards predictive, data-driven imaging aligns with the broader movement towards precision medicine.
However, the deployment of AI systems introduces challenges around data quality, validation and regulation. Total-body PET produces vast datasets that require robust storage and computational infrastructure. Ensuring patient privacy, reproducibility and standardisation across centres will be essential as AI becomes embedded in clinical workflows.
Theranostics and Personalised Medicine
The convergence of PET imaging with targeted radiotherapy—known as theranostics—represents one of the most exciting frontiers in nuclear medicine. By using matched diagnostic and therapeutic isotopes, clinicians can identify suitable patients, deliver precision treatment, and monitor response with quantitative accuracy.
Advances in total-body PET and AI-based dosimetry now enable high-temporal-resolution assessment of radiopharmaceutical distribution across the entire body. This supports a truly personalised approach to therapy planning, ensuring that tumour sites receive an adequate dose while minimising toxicity to healthy tissue.
Recent developments in radiotheranostic tracers extend beyond prostate cancer to include neuroendocrine tumours, breast cancer and even inflammatory diseases. As these approaches mature, PET imaging will play an increasingly central role in treatment planning and follow-up, moving from a diagnostic modality to an integral component of therapy.
Clinical Workflow and Real-World Considerations
While technological progress is impressive, practical challenges remain in bringing these advances to everyday clinical practice. Total-body PET scanners, for instance, involve high acquisition and maintenance costs, as well as the need for specialised training. Centres adopting these systems must develop new workflows for data management, dynamic imaging protocols and quality assurance.
Changes in patient management also continue to influence PET interpretation. A recent study showed that GLP-1 receptor agonists—widely prescribed for diabetes and weight management—can alter tracer uptake patterns in FDG PET/CT scans. This highlights the importance of considering pharmacological factors in image interpretation and underscores the need for close coordination between imaging specialists and clinicians.
In addition, the growing range of tracers necessitates updated clinical guidelines and regulatory frameworks. Approval pathways, reimbursement policies and production logistics will determine how quickly novel tracers reach routine use. Collaboration among academia, industry, and regulatory bodies will be vital to bridging the gap between innovation and clinical implementation.
The UK’s Emerging PET Ecosystem
The UK is positioning itself at the forefront of next-generation PET imaging. The National PET Imaging Platform’s rollout of total-body scanners and its emphasis on standardisation and open data will enable large-scale multi-centre research. For laboratories and radiopharmacies, participation in these networks offers access to advanced equipment, broader clinical collaborations and opportunities for translational research.
With PET procedure volumes rising globally—up by more than 12% in the United States alone in 2024—demand for radiopharmaceutical production and skilled personnel is growing in parallel. This trend places renewed emphasis on workforce development, quality assurance, and infrastructure investment.
For PET chemistry teams, the challenge will be to align tracer development with new imaging capabilities. Total-body scanners require consideration of multi-organ kinetics and long-range quantification, while hybrid systems introduce additional validation steps. At the same time, the shift towards AI-driven workflows offers opportunities to streamline synthesis planning, automate data analysis and improve reproducibility.
Looking Ahead
The latest developments in PET imaging collectively signal a transformation in how molecular information is captured and applied. Total-body PET brings unprecedented sensitivity and temporal resolution. Novel radiotracers expand the reach of molecular imaging into previously inaccessible biological pathways. AI enhances image reconstruction, quantification and therapy planning. Together, these advances are reshaping the landscape of diagnostics and personalised medicine.
For researchers and clinical practitioners, the priority now lies in integration—aligning chemistry, physics, biology and computation into a seamless workflow. For policymakers and funders, the challenge is to ensure that these high-cost technologies deliver measurable clinical benefit. And for those leading radiochemistry and imaging teams, the opportunity is clear: to position PET not merely as a diagnostic tool but as a driver of precision health.
The coming years are likely to see greater emphasis on dynamic, multi-tracer imaging, AI-assisted interpretation, and cross-modality studies combining PET, CT, MRI, and optical techniques. As PET becomes faster, more quantitative and more personalised, it will continue to play a central role in understanding disease and guiding therapy across a widening spectrum of medicine.
Disclaimer
The content in this article is intended for informational and educational purposes only. It does not constitute medical advice, diagnosis, or treatment. Readers should consult qualified healthcare professionals or relevant specialists regarding any medical or technical concerns. Open MedScience and the author make no representations or warranties about the accuracy, completeness, or applicability of the information provided, and accept no liability for any actions taken based on its content.