The most commonly used medical radioisotope in diagnostic procedures is technetium-99m.
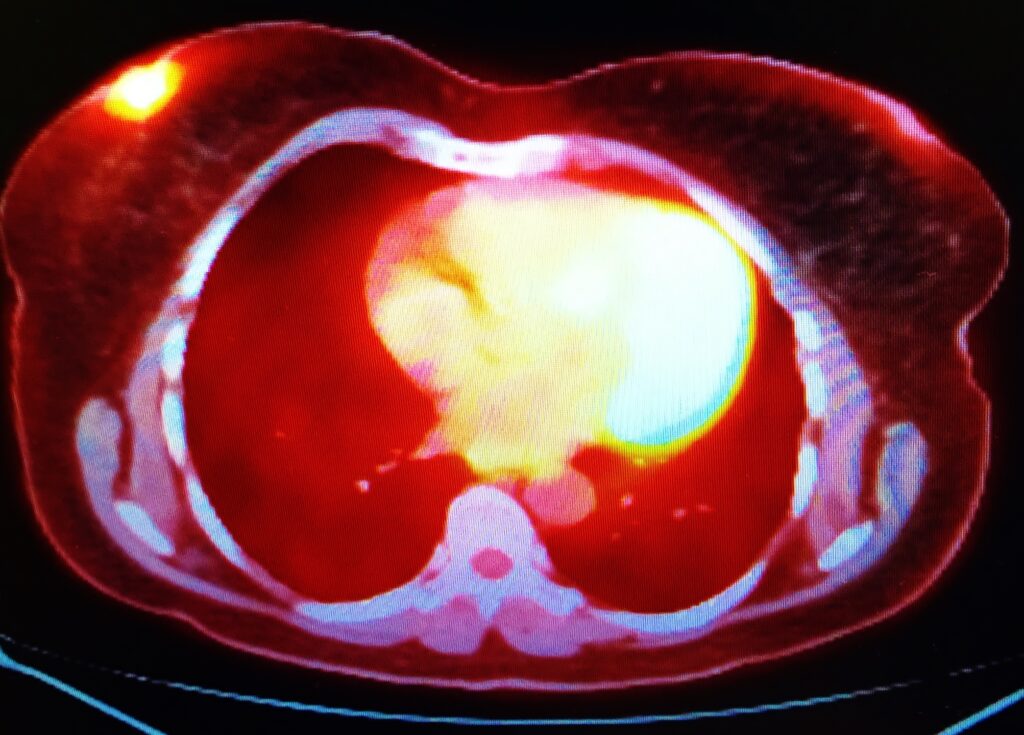
Heart myocardial perfusion imaging with technetium-99m Myoview
The most commonly used medical radioisotope, especially in heart myocardial perfusion, is the metastable nuclear isomer of technetium-99, known as technetium-99m (Tc-99m). This radioisotope was discovered in the 1950s by Walter Tucker and Margaret Greene at the Brookhaven National Laboratory. The first article on the use of technetium as a medical tracer was presented by Powell Richards at the 7th International Electronic and Nuclear Symposium in Rome in June of 1960. Paul Harper was the first person to purchase a technetium-99m generator from the Brookhaven National Laboratory to investigate patient blood flow measurements. During these experiments, he observed the rapid uptake of technetium-99m in the thyroid gland, especially in brain tumours. In 1976, Powell Richards developed the first kit for labelling red blood cells, and the subsequent development was made between 1963 and 1966 in the area of diagnostic imaging.
Currently, technetium-99m is the major workhorse in Nuclear Medicine departments throughout the world. This is because of its gamma-ray energy of about 140 keV, making it suitable for detection. Also, both its physical and biological half-life are very short, leading to rapid removal from the human body after an imaging scan. Technetium-99m only emits gamma radiation in the imaging process. There is no beta emission, which allows for a more precise alignment of imaging detectors.
Technetium-99m is produced by bombarding molybdenum-98 with neutrons. The resultant molybdenum-99 decays with a half-life of 66 hours to the metastable state of technetium. This process permits the production of technetium-99m for medical purposes. Since molybdenum-99 is a fission product of uranium-235 fission, it can be separated from other fission products and then used to generate technetium-99m. For medical purposes, the technetium-99m is used in the form of pertechnetate (chemical formula TcO4–).
Technetium-99m has a half-life of 6.03 hours—gamma emission—which is longer for an electromagnetic decay compared to 10-16 seconds. This emission leads to a long half-life for the excited state and decays to a metastable state assigned by 99m. The dominant decay mode generates a gamma ray at 140.5 keV.
The isotope of choice for routine labelling of kits for diagnostic work is technetium-99m. This radionuclide has a gamma photon emission that can be used with the gamma camera with no associated beta emission, therefore minimising the radiation exposure to the patient. The chemical properties of technetium-99m also mean it binds well to the tracers in the kits. Other radioisotopes are also used, for example, indium-111, iodine-123, selenium-75, and fluorine-18.
Cardiac nuclear medicine imaging
Heart Myocardial Perfusion is a nuclear medicine examination of the heart – the myocardium. This technique can be used to evaluate coronary artery disease (CAD) and cardiac stress. Technetium-99m tetrofosmin (Myoview™) is a radiopharmaceutical with many applications in nuclear medicine to generate cardiac images. These heart myocardial perfusion scans play an essential part in the non-intrusive evaluation of coronary artery disease thereby providing prognostic information regarding potential cardiac events for the patient. New radionuclides are being investigated for myocardial perfusion: particularly the use of rubidium-82. The aim is to reduce radiation exposure to the patient of technetium-99m. A complete heart myocardial perfusion examination can be accomplished under a radiation dose of 3 mSv.
SPECT imaging
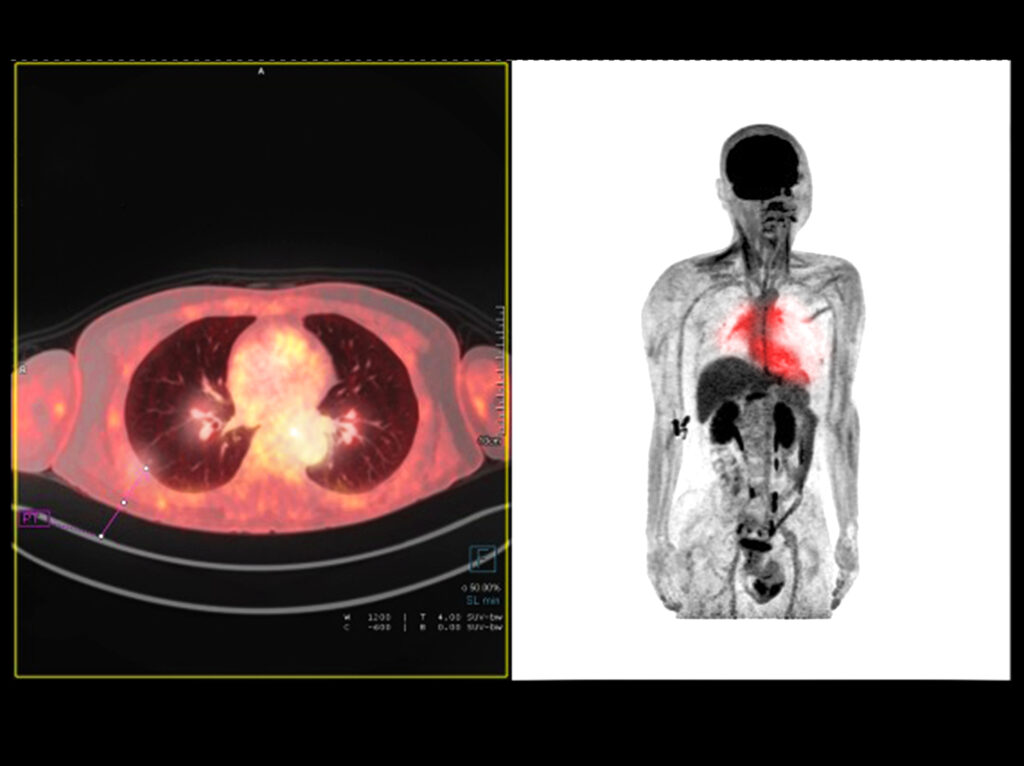
SPECT imaging (single-photon emission computed tomography) is a nuclear medicine tomographic technique which detects gamma radiation through a gamma camera. It is used to generate 3-D images from the distribution of a gamma-emitting radionuclide which is administered via the bloodstream. SPECT emits a gamma array which is in contrast to the positron emitters (fluorine-18) employed by PET (positron emission tomography). These SPECT radiotracers include the technetium-99m. This is a metastable nuclear isomer of technetium-99 compared to indium-111, iodine-123 and thallium-201.
In addition, gaseous xenon-133 has been used in diagnostic inhalation by the usage of a gamma camera. These cameras include the scintillation detector, collimator, sodium iodide crystals and several photomultipliers. Also, imaging of cerebral blood flow is of interest, and several studies are using SPECT radiopharmaceuticals, but these are mostly technetium-99m agents.
These technetium-99m agents contain technetium-99m pertechnetate, technetium-99m DTPA (diethylene-triamine-pentaacetate) used in renal imaging, technetium-99m gluceptate (Tc-GH), technetium-99m exametazime (Tc-HMPAO) and technetium-99m bicisate (Tc-ECD). All gamma radiation emitted from SPECT imaging agents are detected by rotating a gamma camera around the patient to produce 3-D imaging. The generated images undergo several electronic transformations in relation to the distribution of the radiotracer by the application of tomographic techniques.
The radioisotopes used in SPECT imaging have a relatively long half-life; for instance, technetium-99m (t½ = 6 hours), iodine-123 (t½ = 13.22 hours), indium-111 (t½ = 2.8 days), and thallium-201 (t½ = 73 hours). The radionuclide technetium-99m is prepared from molybdenum-99 by using a generator.
These technetium-99m radiopharmaceuticals are relatively cheap compared to PET and fMRI imaging. The main issue with SPECT imaging is reduced spatial resolution. However, the increase in radioactivity of the radiopharmaceutical may contain some safety issues concerning the preparation and dispensing of the radioisotopes.
In some cases, the radionuclide is attached to a specific ligand to produce a radioligand complex. This complex allows the radiopharmaceutical to be transported and localised in a particular part of the body. At the location, the radiopharmaceutical will emit radiation which will be detected by a gamma camera to generate 3-D images.
Hybrid scanners
The diagnostic modalities employed to study the brain include computerised tomography (CT) and fMRI (functional magnetic resonance imaging). This Includes the radiopharmaceuticals used for SPECT and PET. Also for hybrids of PET-CT, SPECT-CT and PET-MRI scanning systems. These hybrid scanning machines are able to identify anatomical construction and contain the diagnostic power to recognise a particular disease pattern. This allows a personalised treatment plan for the patient.
The Brain imaging arsenal contains SPECT and PET radiopharmaceuticals. Recent developments in PET imaging aim to design selective agents for different types of tumours, related cognitive disease and motor indisposition.
Accordingly, SPECT and SPECT-CT imaging of the brain plays a primary role in patient diagnosis. These brain imaging modalities contain photon emitting agents to evaluate epilepsy as well as different cerebrovascular states.
The scanner spatial resolution for clinical SPECT is between 8-12 mm compared to clinical PET 4-6 mm. However, preclinical SPECT ≤ 1 mm compared to preclinical PET 1-2 mm. The clinical gamma cameras have a tomographic spatial decision of approximately 10 mm.
Furthermore, certain preclinical SPECT scanners can provide a submillimetre spatial resolution. Subsequent, further modifications using several pinhole systems can exhibit a spatial analysis below 1 mm. In addition, the clinical and preclinical PET scanners have a spatial separation of 1-2 mm and 4-6 mm relatively.
Developments in PET scanners have generated an amended spatial resolution of approximately 2.5 mm and continuing research is providing a spatial resolution of less than 1 mm. This is achieved by using lutetium orthosilicate (LSO) crystals with applications in the concept of micro-SPECT as a diagnostic screening tool.
Diagnostic imaging kits
Diagnostic imaging kits containing technetium-99m labelled exametazime (Ceretec™) are used to evaluate blood flow within the brain especially after a stroke, epilepsy, Alzheimer’s disease and migraine. This radiopharmaceutical is not charged and lipophilic with a low molecular weight which enables the passage through the blood-brain barrier. Therefore, the maximum uptake of technetium-99m labelled exametazime into the brain occurs within one minute after the in vivo administration and about 7% of the injected dose reaches the brain.
Diagnostic imaging of the heart is made possible using the technetium-99m labelled tetrofosmin (Myoview) and sestamibi (Cardiolite). Both these cardiac imaging agents are used to evaluate patients with myocardial infarction especially locating reversible ischemia and infarcted tissue in the heart.
The digital images are generated when a patient is at rest and in a cardiac stress state from running on a treadmill. During the diagnostic study, the injected dose of technetium-99m is taken up by myocardial tissue and reaches its maximum level in about 5 minutes. After the diagnostic procedure, about 66% of the total injected dose is excreted within 2 days
The patient will receive a dose of Myoview up to 33 mCi followed by a two-dose stress/rest dosing. The standard protocol is usually a 10 mCi dose, then after 4 hours a dose of 30 mCi resulting in Imaging after 15 minutes following injection.
During a ventilation scan, the patient inhales an aerosol of technetium-99m labelled DTPA or radioactive krypton gas. The resulting image shows where air circulates in the lungs. Aerosols of radiolabelled DTPA tend to be used more commonly because aerosol generation devices produce a consistent particle size and technetium-99m is readily available in most radiopharmacy departments.
Disclaimer
The content provided in this article is for general informational and educational purposes only. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your doctor or other qualified health provider with any questions you may have regarding a medical condition or diagnostic procedure. The use of technetium-99m and other radiopharmaceuticals should be carried out by trained professionals in accordance with established clinical guidelines and regulations. Open Medscience does not endorse or promote any specific commercial products or treatments mentioned herein. The information was accurate at the time of publication but may become outdated as new research or clinical practices emerge.
home » diagnostic medical imaging blog » nuclear medicine imaging »